
To find:
The molecular geometries of the given ions.
Answer to Problem 5.28QA
Solution:
The molecular geometries of the ions are (a) linear, (b) tetrahedral, (c) linear, and (d) trigonal pyramidal.
Explanation of Solution
a) SCN-:
i. The Lewis structure:
The total number of valence electrons in a molecule of SCN- is 16.
| Element | Valence electrons | ||
| Symbol | In one atom | Total | |
| S | 1 | 6 | 1 x 6 = 6 |
| C | 1 | 4 | 1 x 4 = 4 |
| N | 1 | 5 | 1 x 5 = 5 |
| Negative charge | 1 | ||
| Valence electrons in SCN- | 16 | ||
C is the central atom because C is least electronegative atom. The Lewis structure of SCN- is drawn by completing the octet of each element present in the molecule by adding lone pairs on it.
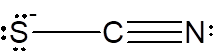
Formula to calculate steric number SN
ii. In the Lewis structure there is one S and one N atom attached to central C atom having no lone pair of electrons. So SN = 2. There is a -1 formal charge on S atom
iii. For SN = 2 and as the bond angle is 180o, molecular geometry is linear.
b) CH3PCl3+:
i. The Lewis structure:
The total number of valence electrons in a molecule of CH3PCl3+ is 32.
| Element | Valence electrons | ||
| Symbol | Number of atoms | In one atom | Total |
| C | 1 | 4 | 1 x 4 = 4 |
| H | 3 | 1 | 3 x 1 = 3 |
| P | 1 | 5 | 1 x 5 = 5 |
| Cl | 3 | 7 | 3 x 7 = 21 |
| Positive charge | -1 | ||
| Valence electrons in CH3PCl3+ | 32 | ||
P is the central atom. The Lewis structure of CH3PCl3+ is drawn by completing the octet of each element present in the molecule by adding lone pairs on it.
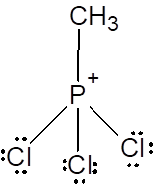
ii. In the Lewis structure there are three chlorine atoms and one CH3 group via C atom bonded to central P atom. So SN = 4. There is +1 formal charge on three O atoms. So SN = 4.
iii. For SN = 4, the molecular geometry is tetrahedral and bond angle is 109.5o.
c) ICl2-:
i. The Lewis structure:
The total number of valence electrons in a molecule of ICl2- is 22.
| Element | Valence electrons | ||
| Symbol | Number of atoms | In one atom | Total |
| I | 1 | 7 | 1 x 7 = 7 |
| Cl | 2 | 7 | 2 x 7 = 14 |
| Negative charge | 1 | ||
| Valence electrons in ICl2- | 22 | ||
I is the central atom because I is least electronegative atom. The Lewis structure of ICl2- is drawn by completing the octet of each element present in the molecule by adding lone pairs on it.
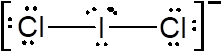
ii. In the Lewis structure there are two oxygen atoms bonded to central I atom. There are three lone pair on I atom with -1 formal charge. So SN = 5.
iii. For SN = 5, the electron geometry is trigonal bipyramidal. As all lone pairs are at equatorial position to minimize the repulsion the molecular geometry is linear.
d) PO33-:
i. The Lewis structure:
The total number of valence electrons in a molecule of PO33- is 26.
| Element | Valence electrons | ||
| Symbol | Number of atoms | In one atom | Total |
| P | 1 | 5 | 1 x 5 = 5 |
| O | 3 | 6 | 3 x 6 = 18 |
| Negative charge | 3 | ||
| Valence electrons in PO33- | 26 | ||
P is the central atom because P is least electronegative atom. The Lewis structure of PO33- is drawn by completing the octet of each element present in the molecule by adding lone pairs on it.
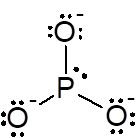
ii. In the Lewis structure there are three O atoms bonded to central P atom. There is one lone pair on P atom. So SN = 4.
iii. If SN = 4, the electron geometry is tetrahedral but as there is one lone pair on P atom and it is bonded to three O atoms the geometry is trigonal pyramidal.
Conclusion:
The molecular geometries of the ions are (a) linear, (b) tetrahedral, (c) linear, and (d) trigonal pyramidal.
Want to see more full solutions like this?
Chapter 5 Solutions
Chemistry: An Atoms-Focused Approach (Second Edition)
- 21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward
- 4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forwardIn the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forwardIndicate the processes in the dismutation of Cu2O.arrow_forward
- 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 Potential Energy (kJ) 600 400 200 0 -200- -400 -600- -800 (i) Cl₂ (g) + Pt(s) → 2Cl (g) + Pt(s) (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) Ea = 1550 kJ Ea = 2240 kJ (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2350 kJ AH=-950 kJ ΔΗ = 575 ΚΙ AH=-825 kJ a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ Reaction Progress b. What is the overall chemical equation? c. What is the overall change in enthalpy for the above chemical reaction? d. What is the overall amount of activation energy for the above chemical reaction? e. Which reaction intermediate would be considered a catalyst (if any) and why? f. If you were to add 2700kJ of energy to the reaction (e.g. 2700 kl of heat or electricity), would you be able to make the reaction reverse itself (i.e. have…arrow_forwarddraw the enolate anion and the carbonyl that would be needed to make this product through an aldol addition reaction.arrow_forwardDraw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forward
- Draw the Michael adduct and final product of the Robinson annulation reaction. Ignore inorganic byproductsarrow_forwardPost Lab Questions. 1) Draw the mechanism of your Diels-Alder cycloaddition. 2) Only one isomer of product is formed in the Diels-Alder cycloaddition. Why? 3) Imagine that you used isoprene as diene - in that case you don't have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here. 4) This does not hold for other dienes. Draw the exo and endo products of the reaction of cyclohexadiene with maleic anhydride. Make sure you label your answers properly as endo or exo. 100 °C Xylenes ??? 5) Calculate the process mass intensity for your specific reaction (make sure to use your actual amounts of reagent).arrow_forwardIndicate the product(s) A, B C and D that are formed in the reaction: H + NH-NH-CH [A+B] [C+D] hydrazonesarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





