
To find:
The Bond order of N2+, O2+, C2+, and Br22- from the MO theory and predict the bond orders.
Answer to Problem 5.103QA
Solution:
1) Bond order of N2+ = 2.5
2) Bond order of O2+ = 2.5
3) Bond order of C2+ = 1.5
4) Bond order of Br22- = 0
All species with nonzero bond order may exist, therefore, N2+, O2+ and C2+ may exist.
Explanation of Solution
The Molecular orbital diagram of N2+, O2+, C2+, and Br22- are below
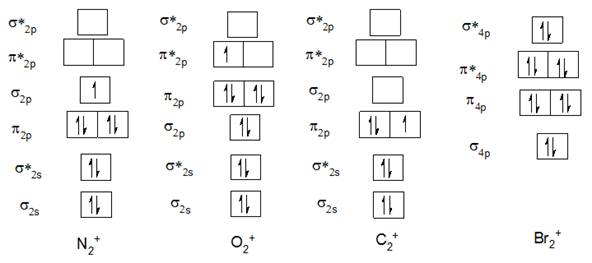
1) Bond order of N2+
:
Electronic configuration of N2+ is
2) Bond order of O2+:
Electronic configuration of O2+ is
3) Bond order of C2+:
Electronic configuration of C2+ is
4) Bond order of Br22- :
In case of Br22- after 4s there is 3d subshell which makes the orbital diagram complicated and as it is completely filled, it is going to cancel out with bonding and antibonding electrons;hence, it is neglected in this case. Valence electronic configuration of Br22- is
The bond order of Br22- is 0 because bonding and antibonding electrons are equal.
All species with nonzero bond order may exist, therefore, N2+, O2+ and C2+ may exist.
Conclusion:
The bond order of the given molecular ions is calculated from the MO diagram. It is used to check the existence of species from the bond order.
Want to see more full solutions like this?
Chapter 5 Solutions
Chemistry: An Atoms-Focused Approach (Second Edition)
- I have a 2 mil plastic film that degrades in 22 days at 88C and 153 days at 61C what is the predicted theoretical degradation at 47C?arrow_forwardno ai walkthrougharrow_forwardI have a 2 mil plastic film that degrades after 22 days at 88C and at 61C takes 153 days. What is the failure at 47C in days.arrow_forward
- If a 5 film plastic film degraded in 30 days at 35C and the same film degraded in 10 days at 55 C and 2 days at 65C what would the predicted life time be at 22C for the same film?arrow_forwardno Ai walkthroughsarrow_forwardI have a aqueous solution (175 ml) of iridium trichloride containing 8,750 ppm Iridium by ICP OES analysis. What is the percent concentration of Iridium trichloride in aquous solution and provide the concentration in moles per liter, percentage by weight.arrow_forward
- no Ai walkthroughsarrow_forward136 PRACTICAL SPECTROSCOPY Compound 78 is a high-boiling liquid (boiling point 189° C) that contains halogen, but will not react with alkoxides to yield an halogen. ether. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 35.32; H, 2.47; contains BC Spectral Data: doublet, 137.4 ppm; doublet, 130.1 ppm; doublet, 127.4 ppm; singlet, 97.3 ppm Absorbance Mass Spectrum Intensity 77 77 204 M + 128 40 60 80 100 120 140 160 180 m/e 200 220 280 240 260 300 Infrared Spectrum Wave Number, cm -1 4000 3000 2500 2000 1500 1300 1200 1100 1000 900 800 700 3 6 7 8 9 10 12 13 15 Wavelength, microns 'H NMR wwwww 5 Structure: www ppm, & ©2000 Brooks/Cole Publishing Com-arrow_forwardno Ai walkthroughsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





