
Concept explainers
(a)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image. But not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are
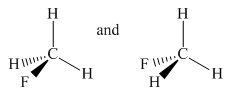
In the above pair, both molecules have carbon as the central atom with one fluorine atom and three hydrogen atoms as an attachment. In the left molecule, fluorine is pointing towards the observer and one hydrogen atom is pointing away from the observer while in the second molecule fluorine is pointing away from the observer and one hydrogen atom is pointing towards the observer.
To check the molecule in different orientation the molecule present at right is rotated.
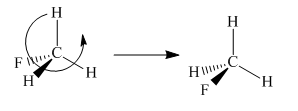
The molecule after the rotation has the exact similar orientation of the atoms as the left molecule in the given pair i.e. all atoms are lined up perfectly.
Thus both the molecules in the given pair are superimposable.
All atoms of both molecules are lined perfectly hence superimposable.
(b)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are
In given pair, both molecules are mirror images of each other. If the molecule at right is rotated, it gives the molecule exactly similar to the left one.
Thus both the molecules in the given pair are superimposable.
All atoms of both the molecules are lined perfectly hence superimposable.
(c)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are nonsuperimposable.
Explanation of Solution
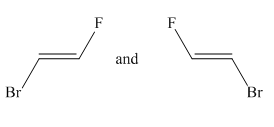
Molecules in the given pair are:
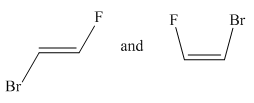
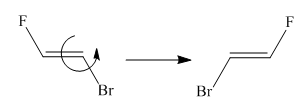
Two molecules given in the pair are the cis-trans isomers of the same molecule. To get the trans isomer from cis, rotation is needed around the double bond which is restricted. Hence the atoms in both molecules are not lined up perfectly, thus the given two molecules are nonsuperimposable.
All atoms of both molecules are not lined perfectly hence nonsuperimposable.
(d)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
The molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are:
![]()
Two molecules given in pairs are the mirror images of each other. If one of the two given molecules is rotated around the central carbon atom it gives the exact similar molecule as another.
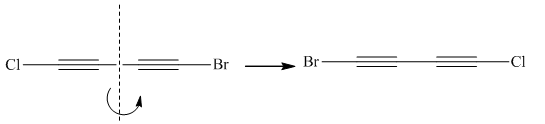
Rotation of the molecule gives the molecule with a similar orientation as another molecule, thus two molecules are superimposable.
All atoms of both molecules are lined perfectly, hence superimposable.
(e)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
The molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are:
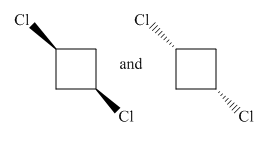
Both molecules in the given pair are isomers of the same molecules thus have the same attachments of the molecule with different orientations. Two chlorine atoms in the left molecule are pointing towards the observer while two chlorine atoms in the right molecule are pointing away from the observer. If one of the molecule rotates with
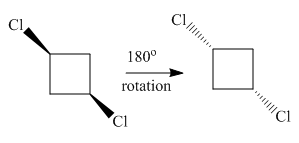
The molecule after the rotation of the left molecule has the exact similar orientation as the molecule at right, all atoms of both molecules are lined perfectly representing the molecules in the given pair are superimposable.
All atoms of both molecules are lined perfectly hence superimposable.
(f)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image, but not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are nonsuperimposable.
Explanation of Solution
Molecules in the given pair are
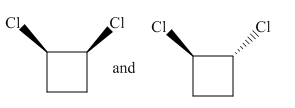
In the molecule at the left, both chlorine atoms are pointing towards the observer while in the molecule at right one chlorine atom is pointing towards the observer and one is pointing away from the observer. To get a similar orientation, rotation of the atom with the ring is needed which is restricted. Thus there is no orientation of both molecules lined perfectly, representing the molecules in the given pair are nonsuperimposable.
All atoms of both molecules are not lined perfectly, hence nonsuperimposable.
(g)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
Molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image. But not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are superimposable.
Explanation of Solution
Molecules in the given pair are
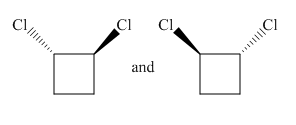
In the given pair both molecules are mirror images of each other having one chlorine atom pointing towards the observer and another pointing away from the observer.
The complete rotation of
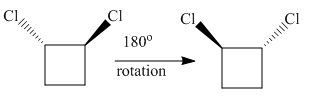
The molecule after the rotation of the left molecule has the exact similar orientation as the molecule at right, all atoms of both molecules are lined perfectly representing the molecules in the given pair are superimposable.
All atoms of both molecules are lined perfectly hence superimposable.
(h)
Interpretation:
Whether the molecules in the given pair are superimposable or nonsuperimposable is to be determined.
Concept introduction:
The molecules are nonsuperimposable if there is no orientation in which all atoms of both molecules can be lined up perfectly (i.e., superimposed). Every molecule can have a mirror image. But not every molecule can have a nonsuperimposable mirror image.
Answer to Problem 5.1P
The given two molecules are nonsuperimposable.
Explanation of Solution
Molecules in the given pair are
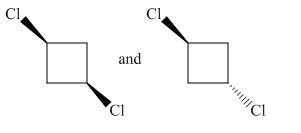
In the molecule at left both chlorine atoms are pointing towards the observer while in the molecule at right one chlorine atom is pointing towards the observer and one is pointing away from the observer. To get a similar orientation, rotation of the atom with the ring is needed which is restricted. Thus no orientation of both molecules lined perfectly, representing the molecules in the given pair are nonsuperimposable.
All atoms of both molecules are not lined perfectly hence nonsuperimposable.
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- > H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forwardno Ai walkthroughsarrow_forwardThe answer is shown. What is the reaction mechanism to arrive at the answer?arrow_forward
- no Ai walkthroughsarrow_forwardConsider the following nucleophilic substitution reaction. The compound listed above the arrow is the solvent for the reaction. If nothing is listed over the arrow, then the nucleophile is also the solvent for the reaction. Part 1 of 2 Br CH,CN + I¯ What is the correct mechanism for the reaction? Select the single best answer. @SN2 ○ SN 1 Part: 1/2 Part 2 of 2 Draw the products for the reaction. Include both the major organic product and the inorganic product. If more than one stereoisomer is possible, draw only one stereoisomer. Include stereochemistry where relevant. Click and drag to start drawing a structure. X હૈarrow_forward20.33 Think-Pair-Share (a) Rank the following dienes and dienophiles in order of increasing reactivity in the Diels-Alder reaction. (i) CO₂Et (ii) COEt || CO₂Et MeO MeO (b) Draw the product that results from the most reactive diene and most reactive dienophile shown in part (a). (c) Draw a depiction of the orbital overlap involved in the pericyclic reaction that oc- curs between the diene and dienophile in part (b). (d) Is the major product formed in part (b) the endo or exo configuration? Explain your reasoning.arrow_forward
- 20.40 The following compound undergoes an intramolecular Diels-Alder reaction to give a tricyclic product. Propose a structural formula for the product. CN heat An intramolecular Diels-Alder adductarrow_forwardWhat is the reaction mechanism for this? Can this even be done without a base?arrow_forwardWhat is the reaction mechanism for this?arrow_forward
- What is the reaction mechanism for this?arrow_forwardWhat is the reaction mechanism for this?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. + Drawing Arrows CH3ONA, CH3OH heat : Br:O Na → H H Br Na + H H H H H :0: .H + Undo Reset Done Q CH3 Drag To Pan +arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
