
Concept explainers
(a)
Interpretation:The isomeric relationship between cyclopropylcyclopentane and cyclobutylcyclobutane should beidentified.
Concept introduction:Chiral carbon is any stereocenter attached to four different alkyl substituents. If any two of the substituent happen to be similar the center is regarded as achiral.
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold similar mirror image relationships.
The molecular models of

The two compounds constitute a pair of constitutional isomers. These kinds of isomers are formed by cleavage and replacement of bonds, unlike the conformational isomers that result due to rotation around the bond.
(a)
Explanation of Solution
Structures of cyclopropylcyclopentane and cyclobutylcyclobutane are illustrated as follows:

The molecular formula for both cyclopropylcyclopentane and cyclobutylcyclobutane is
(b)
Interpretation: The isomeric relationship among
Concept introduction: Chiral carbon is any stereocenter attached to four different alkyl substituents. If any two of the substituent happen to be similar the center is regarded as achiral.
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold similar mirror image relationships.
The molecular models of

The two compounds constitute a pair of constitutional isomers. These kinds of isomers are formed by cleavage and replacement of bonds, unlike the conformational isomers that result due to rotation around the bond.
(b)
Explanation of Solution
Structures of
are illustrated as follows:
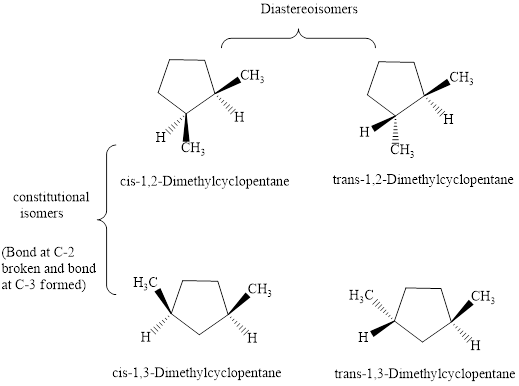
Since cis and trans form represent diastereoisomers therefore
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry: Structure and Function
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





