![OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months)](https://www.bartleby.com/isbn_cover_images/9781305864900/9781305864900_largeCoverImage.jpg)
Concept explainers
The graph here represents the distribution of molecular speeds of hydrogen and neon at 200 K.
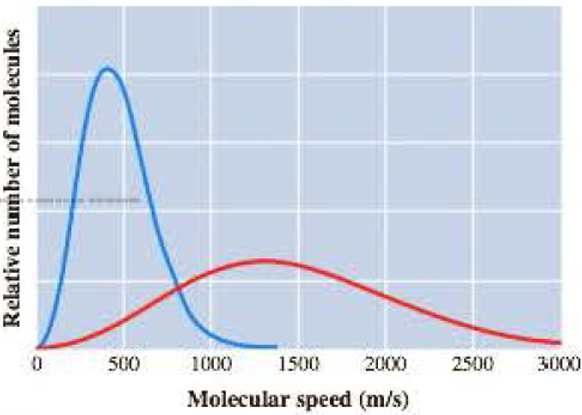
- a Match each curve to the appropriate gas.
- b Calculate the rms speed (in m/s) for each of the gases at 200 K.
- c Which of the gases would you expect to have the greater effusion rate at 200 K? Justify your answer.
- d Calculate the temperature at which the rms speed of the hydrogen gas would equal the rms speed of the neon at 200 K.
(a)
Interpretation:
The curve in the given graph must be matched with the appropriate gas.
Answer to Problem 5.136QP
The taller and narrow curve represents neon atoms
The flatter and wider curve represents hydrogen molecules
Explanation of Solution
Given,
The given graph represents the distribution of molecular speeds of hydrogen and neon at
Matching of given curves with appropriate gases:
In the given graph, the taller and narrow curve whose maximum falls near
The flatter and wider curve whose maximum falls near
The taller and narrow curve matches with neon atoms
The flatter and wider curve matches with hydrogen molecules
(b)
Interpretation:
The rms speed (in
Concept Introduction:
Root-mean-square (rms):
The root-mean-square molecular speed (
Where,
Answer to Problem 5.136QP
The rms speed of neon gas at
The rms speed of hydrogen gas at
Explanation of Solution
Given,
The given graph represents the distribution of molecular speeds of hydrogen and neon at
Calculation of rms speed:
The rms speed of neon is calculated as follows,
The rms speed of hydrogen gas is calculated as follows,
The rms speed of neon gas at
The rms speed of hydrogen gas at
(c)
Interpretation:
The gas that has greater effusion rate at
Concept Introduction:
Graham’s law of effusion:
Answer to Problem 5.136QP
The gas that has greater effusion rate at
Explanation of Solution
Given,
The given graph represents the distribution of molecular speeds of hydrogen and neon at
Gas possessing greater effusion rate:
The rates of effusion are directly related to the rms speed.
Greater the rms speed, greater is the rate of effusion.
Since the rms speed of hydrogen is greater than neon, hydrogen gas will have greater effusion rate.
In the container, the fast moving molecules collide with the holes of the container more often and hence have a higher effusion probability.
The gas that has greater effusion rate at
(d)
Interpretation:
The temperature at which the rms speed of the hydrogen gas equals the rms speed of neon gas at
Concept Introduction:
Root-mean-square (rms):
The root-mean-square molecular speed (
Where,
Answer to Problem 5.136QP
The temperature at which the rms speed of the hydrogen gas equals the rms speed of neon gas at 200 K is
Explanation of Solution
Given,
The given graph represents the distribution of molecular speeds of hydrogen and neon at
Temperature calculation:
The temperature equaling the rms speed of neon is calculated from root mean square equation as follows,
The temperature at which the rms speed of the hydrogen gas equals the rms speed of neon gas at
Want to see more full solutions like this?
Chapter 5 Solutions
OWLv2 with Student Solutions Manual eBook for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 4 terms (24 months)
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Organic Chemistry
General, Organic, and Biological Chemistry - 4th edition
Organic Chemistry (8th Edition)
- For each of the following, indicate whether the arrow pushes are valid. Do we break any rules via the arrows? If not, indicate what is incorrect. Hint: Draw the product of the arrow and see if you still have a valid structure. a. b. N OH C. H N + H d. e. f. مه N COHarrow_forwardDecide which is the most acidic proton (H) in the following compounds. Which one can be removed most easily? a) Ha Нь b) Ha Нь c) CI CI Cl Ha Ньarrow_forwardProvide all of the possible resonanse structures for the following compounds. Indicate which is the major contributor when applicable. Show your arrow pushing. a) H+ O: b) c) : N :O : : 0 d) e) Оarrow_forward
- Draw e arrows between the following resonance structures: a) b) : 0: :0: c) :0: N t : 0: بار Narrow_forwardDraw the major substitution products you would expect for the reaction shown below. If substitution would not occur at a significant rate under these conditions, check the box underneath the drawing area instead. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. Cl Substitution will not occur at a significant rate. Explanation Check :☐ O-CH + Х Click and drag to start drawing a structure.arrow_forwardDraw the major substitution products you would expect for the reaction shown below. If substitution would not occur at a significant rate under these conditions, check the box underneath the drawing area instead. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. Cl C O Substitution will not occur at a significant rate. Explanation Check + O-CH3 Х Click and drag to start drawing a structure.arrow_forward
- ✓ aw the major substitution products you would expect for the reaction shown below. If substitution would not occur at a significant rate under these conditions, check the box underneath the drawing area instead. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. C Cl HO–CH O Substitution will not occur at a significant rate. Explanation Check -3 ☐ : + D Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Cearrow_forwardPlease correct answer and don't used hand raitingarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Determine whether the following reaction is an example of a nucleophilic substitution reaction: Br OH HO 2 -- Molecule A Molecule B + Br 义 ollo 18 Is this a nucleophilic substitution reaction? If this is a nucleophilic substitution reaction, answer the remaining questions in this table. Which of the reactants is referred to as the nucleophile in this reaction? Which of the reactants is referred to as the organic substrate in this reaction? Use a ŏ + symbol to label the electrophilic carbon that is attacked during the substitution. Highlight the leaving group on the appropriate reactant. ◇ Yes O No O Molecule A Molecule B Molecule A Molecule B टेarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





