
Student Solutions Manual for Zumdahl/Zumdahl/DeCoste?s Chemistry, 10th Edition
10th Edition
ISBN: 9781305957510
Author: ZUMDAHL, Steven S.; Zumdahl, Susan A.; DeCoste, Donald J.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 43E
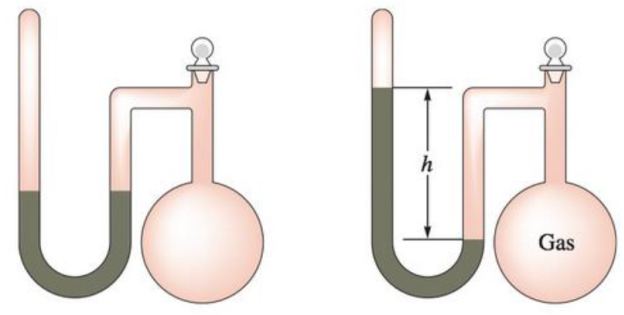
A sealed-tube manometer (as shown below) can be used to measure pressures below atmospheric pressure. The tube above the mercury is evacuated. When there is a vacuum in the flask, the mercury levels in both arms of the U-tube are equal. If a gaseous sample is introduced into the flask, the mercury levels are different. The difference h is a measure of the pressure of the gas inside the flask. If h is equal to 6.5 cm, calcúlate the pressure in the flask in torr, pascals, and atmospheres.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Seee the attached ima
Please see the attached image.
Please see the attached image.
Chapter 5 Solutions
Student Solutions Manual for Zumdahl/Zumdahl/DeCoste?s Chemistry, 10th Edition
Ch. 5 - Prob. 1RQCh. 5 - Prob. 2RQCh. 5 - Prob. 3RQCh. 5 - Prob. 4RQCh. 5 - Prob. 5RQCh. 5 - Prob. 6RQCh. 5 - Prob. 7RQCh. 5 - Prob. 8RQCh. 5 - Prob. 9RQCh. 5 - Why do real gases not always behave ideally? Under...
Ch. 5 - Prob. 3ALQCh. 5 - Prob. 4ALQCh. 5 - Prob. 6ALQCh. 5 - Prob. 8ALQCh. 5 - Prob. 11ALQCh. 5 - Prob. 12ALQCh. 5 - Prob. 13ALQCh. 5 - Prob. 14ALQCh. 5 - Prob. 17ALQCh. 5 - Prob. 18ALQCh. 5 - Draw molecular-level views that show the...Ch. 5 - Prob. 22QCh. 5 - Prob. 23QCh. 5 - Prob. 24QCh. 5 - Prob. 25QCh. 5 - As weather balloons rise from the earths surface,...Ch. 5 - Prob. 27QCh. 5 - Consider two different containers, each filled...Ch. 5 - Prob. 29QCh. 5 - Prob. 32QCh. 5 - Prob. 33QCh. 5 - Prob. 34QCh. 5 - Prob. 35QCh. 5 - Prob. 36QCh. 5 - Prob. 37QCh. 5 - Without looking at a table of values, which of the...Ch. 5 - Prob. 39QCh. 5 - Prob. 40QCh. 5 - Prob. 41ECh. 5 - Prob. 42ECh. 5 - A sealed-tube manometer (as shown below) can be...Ch. 5 - Prob. 44ECh. 5 - A diagram for an open-tube manometer is shown...Ch. 5 - Prob. 46ECh. 5 - Prob. 47ECh. 5 - Prob. 48ECh. 5 - An 11.2-L sample of gas is determined to contain...Ch. 5 - Prob. 50ECh. 5 - Prob. 51ECh. 5 - Prob. 52ECh. 5 - Prob. 53ECh. 5 - Prob. 54ECh. 5 - The Steel reaction vessel of a bomb calorimeter,...Ch. 5 - A 5.0-L flask contains 0.60 g O2 at a temperature...Ch. 5 - Prob. 57ECh. 5 - A person accidentally swallows a drop of liquid...Ch. 5 - Prob. 59ECh. 5 - N2O is a gas commonly used to help sedate patients...Ch. 5 - A gas sample containing 1.50 moles at 25C exerts a...Ch. 5 - Prob. 62ECh. 5 - Prob. 63ECh. 5 - What will be the effect on the volume of an ideal...Ch. 5 - Prob. 65ECh. 5 - Prob. 66ECh. 5 - An ideal gas is contained in a cylinder with a...Ch. 5 - Prob. 68ECh. 5 - A sealed balloon is filled with 1.00 L helium at...Ch. 5 - Prob. 70ECh. 5 - Consider the following reaction:...Ch. 5 - A student adds 4.00 g of dry ice (solid CO2) to an...Ch. 5 - Air bags are activated when a severe impact causes...Ch. 5 - Concentrated hydrogen peroxide solutions are...Ch. 5 - In 1897 the Swedish explorer Andre tried to reach...Ch. 5 - Sulfur trioxide, SO3, is produced in enormous...Ch. 5 - A 15.0-L rigid container was charged with 0.500...Ch. 5 - An important process for the production of...Ch. 5 - Consider the reaction between 50.0 mL liquid...Ch. 5 - Urea (H2NCONH2) is used extensively as a nitrogen...Ch. 5 - Prob. 81ECh. 5 - Prob. 82ECh. 5 - Prob. 83ECh. 5 - A compound has the empirical formula CHCl. A...Ch. 5 - Prob. 85ECh. 5 - Silicon tetrachloride (SiCl4) and trichlorosilane...Ch. 5 - Prob. 87ECh. 5 - Prob. 88ECh. 5 - For scuba dives below 150 ft, helium is often used...Ch. 5 - Prob. 90ECh. 5 - Consider the flasks in the following diagram. What...Ch. 5 - Consider the flask apparatus in Exercise 85, which...Ch. 5 - Prob. 93ECh. 5 - At 0C a 1.0-L flask contains 5.0 102 mole of N2,...Ch. 5 - A mixture of cyclopropane and oxygen is sometimes...Ch. 5 - Prob. 96ECh. 5 - Prob. 97ECh. 5 - A tank contains a mixture of 52.5 g oxygen gas and...Ch. 5 - Prob. 99ECh. 5 - Helium is collected over water at 25C and 1.00 atm...Ch. 5 - At elevated temperatures, sodium chlorate...Ch. 5 - Xenon and fluorine will react to form binary...Ch. 5 - Methanol (CH3OH) can be produced by the following...Ch. 5 - In the Mthode Champenoise, grape juice is...Ch. 5 - Hydrogen azide, HN3, decomposes on heating by the...Ch. 5 - Prob. 106ECh. 5 - Prob. 107ECh. 5 - The oxides of Group 2A metals (symbolized by M...Ch. 5 - Prob. 109ECh. 5 - Prob. 110ECh. 5 - Prob. 111ECh. 5 - Prob. 112ECh. 5 - Prob. 113ECh. 5 - Prob. 114ECh. 5 - Prob. 115ECh. 5 - Prob. 116ECh. 5 - Prob. 117ECh. 5 - Prob. 118ECh. 5 - Prob. 119ECh. 5 - Prob. 120ECh. 5 - Prob. 121ECh. 5 - Prob. 122ECh. 5 - Prob. 123ECh. 5 - Prob. 124ECh. 5 - Use the data in Table 84 to calculate the partial...Ch. 5 - Prob. 126ECh. 5 - Prob. 127ECh. 5 - Prob. 128ECh. 5 - Prob. 129ECh. 5 - Prob. 130ECh. 5 - Prob. 131AECh. 5 - At STP, 1.0 L Br2 reacts completely with 3.0 L F2,...Ch. 5 - Prob. 133AECh. 5 - Prob. 134AECh. 5 - Prob. 135AECh. 5 - Cyclopropane, a gas that when mixed with oxygen is...Ch. 5 - The nitrogen content of organic compounds can be...Ch. 5 - Hyperbaric oxygen therapy is used to treat...Ch. 5 - A 15.0L tank is filled with H2 to a pressure of...Ch. 5 - A spherical glass container of unknown volume...Ch. 5 - Prob. 141AECh. 5 - A 20.0L stainless steel container at 25C was...Ch. 5 - Metallic molybdenum can be produced from the...Ch. 5 - Prob. 144AECh. 5 - Prob. 145AECh. 5 - One of the chemical controversies of the...Ch. 5 - An organic compound contains C, H, N, and O....Ch. 5 - Prob. 148AECh. 5 - Prob. 149CWPCh. 5 - Prob. 150CWPCh. 5 - A certain flexible weather balloon contains helium...Ch. 5 - A large flask with a volume of 936 mL is evacuated...Ch. 5 - A 20.0L nickel container was charged with 0.859...Ch. 5 - Consider the unbalanced chemical equation below:...Ch. 5 - Prob. 155CWPCh. 5 - Which of the following statements is(are) true? a....Ch. 5 - A chemist weighed out 5.14 g of a mixture...Ch. 5 - A mixture of chromium and zinc weighing 0.362 g...Ch. 5 - Prob. 159CPCh. 5 - You have an equimolar mixture of the gases SO2 and...Ch. 5 - Methane (CH4) gas flows into a combustion chamber...Ch. 5 - Prob. 162CPCh. 5 - Prob. 163CPCh. 5 - Prob. 164CPCh. 5 - You have a helium balloon at 1.00 atm and 25C. You...Ch. 5 - We state that the ideal gas law tends to hold best...Ch. 5 - You are given an unknown gaseous binary compound...Ch. 5 - Prob. 168CPCh. 5 - Prob. 170IPCh. 5 - In the presence of nitric acid, UO2+ undergoes a...Ch. 5 - Silane, SiH4, is the silicon analogue of methane,...Ch. 5 - Prob. 173IPCh. 5 - Prob. 174IPCh. 5 - Prob. 175MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY