
Concept explainers
(a)
Interpretation:
The condensed structural formula and systematic name should be given for the molecular formula of
Concept introduction:
A condensed structural formula is a system of writing organic structures in a line of text.
Isomer: A molecule having the same molecular formula but with different chemical structure is called isomer.
Constitutional Isomers: A molecule having same molecular formula with different structural formulas (Difference in the connectivity of the molecule is called constitutional isomer).
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry).IUPAC name consists of three parts, namely Prefix, suffix and root word.
Prefix- Represents the substituent present in the molecule and its position in the root name.
Suffix- Denotes the presence of
Root word - Represents the longest continuous carbon skeleton of the organic molecule.
Alkenes are a class of hydrocarbons. The carbon-carbon double bond is called as alkenes and it is also called as olefins.
(b)
Interpretation:
The E and Z isomer of the alkene has to be identified.
Concept introduction:
E and Z isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
Example:
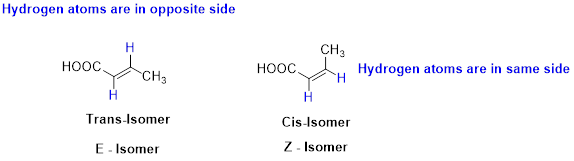
(c)
Interpretation:
The most stable alkene has to be identified.
Concept Introduction:
Stability of alkene:
Stability of alkene depends on the following factors,
The smallest heat hydrogenation of alkene is more stable. The number of hydrogens bonded to its
Stability of Cis–trans alkene:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
In cis alkene, molecule are close to each other, hence electron clouds interfere each other therefore strain in the molecule so cis alkene is less stable. Whereas molecule is away from each other, hence electron clouds are will not interfere each other therefore less strain in the molecule, so trans alkene is stable.
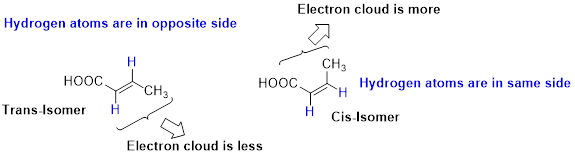
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
- Provide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forward
- Indicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forwardFeedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forwardIf I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning


