
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 22PP
Practice Problem 5.22
Write three-dimensional formulas for all of the stereoisomers of each of the following compounds. Label pairs of enantiomers and label meso compounds.
(a)
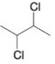
(b)
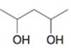
(c)
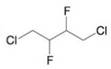
(d)
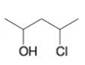
(e)
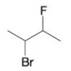
(f)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Construct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)
please help with hw
help me solve this hw
Chapter 5 Solutions
Organic Chemistry
Ch. 5 - Prob. 1PPCh. 5 - Prob. 2PPCh. 5 - Prob. 3PPCh. 5 - Prob. 4PPCh. 5 - Prob. 5PPCh. 5 - Prob. 6PPCh. 5 - Prob. 7PPCh. 5 - Practice Problem 5.8 Write three-dimensional...Ch. 5 - Prob. 9PPCh. 5 - Prob. 10PP
Ch. 5 - Practice Problem 5.11 List the substituents in...Ch. 5 - Prob. 12PPCh. 5 - Practice Problem 5.13 Tell whether the two...Ch. 5 - Prob. 14PPCh. 5 - Prob. 15PPCh. 5 - Prob. 16PPCh. 5 - Prob. 17PPCh. 5 - Prob. 18PPCh. 5 - Prob. 19PPCh. 5 - Prob. 20PPCh. 5 - Practice Problem 5.21 The following are formulas...Ch. 5 - Practice Problem 5.22 Write three-dimensional...Ch. 5 - Prob. 23PPCh. 5 - Practice Problem 5.24 Give names chat include (R)...Ch. 5 - Prob. 25PPCh. 5 - Prob. 26PPCh. 5 - Prob. 27PPCh. 5 - Prob. 28PPCh. 5 - Practice Problem 5.29 Write formulas for all of...Ch. 5 - Prob. 30PPCh. 5 - Prob. 31PPCh. 5 - Prob. 32PPCh. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - 5.35 Designate the (R) or (S) configuration at...Ch. 5 - Prob. 36PCh. 5 - (a) Write the structure of...Ch. 5 - Shown below are Newman projection formulas for...Ch. 5 - 5.39 Write appropriate structural formulas...Ch. 5 - Discuss whether each of the following compounds...Ch. 5 - Prob. 42PCh. 5 - Prob. 43PCh. 5 - Compound F has the molecular formula C5H8 and is...Ch. 5 - Prob. 45PCh. 5 - Prob. 46PCh. 5 - Prob. 47PCh. 5 - For the following molecule, draw its enantiomer as...Ch. 5 - 5.49 (Use models to solve this...Ch. 5 - 5.50 (Use models to solve this...Ch. 5 - (Use models co solve this problem.) Write...Ch. 5 - 5.52 Tartaric acid was an important compound in...Ch. 5 - Prob. 53PCh. 5 - Prob. 54PCh. 5 - Prob. 55PCh. 5 - Prob. 1LGPCh. 5 - Prob. 2LGPCh. 5 - Prob. 3LGPCh. 5 - Prob. 2QCh. 5 - Prob. 3QCh. 5 - Prob. 4QCh. 5 - Select the words that best describe what happens...Ch. 5 - Prob. 6QCh. 5 - Prob. 7QCh. 5 - Prob. 8QCh. 5 - 5.9 Which is untrue about the following...Ch. 5 - Prob. 10Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
In Fig. 12-82, a uniform beam of length 12.0 m is supported by a horizontal cable and a hinge at angle = 50.0....
Fundamentals of Physics Extended
How does the partial pressure of oxygen change as altitude changes?
Principles of Anatomy and Physiology
A source of electromagnetic radiation produces infrared light. Which of the following could be the wavelength ...
Chemistry: The Central Science (14th Edition)
14. A light flashes at position x = 0 m. One microsecond later, a light flashes at position x = 1000 m. In a se...
College Physics: A Strategic Approach (3rd Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
6.1 State the number of electrons that be must be lost by atoms of each of the following to achieve a stable el...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly explain chemical potential.arrow_forwardReason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License