
One of the important ideas of 
- Using this device, what measurements would you need to make to test your hypothesis?
- What equations would you use in analyzing your experiment?
- Do you think you could obtain a reasonable result from a single experiment? Why or why not?
- In what way could the precision of your instruments affect the conclusions that you make?
- List ways that you could modify the equipment to improve the data you obtain if you were performing this experiment today instead of 180 years ago.
- Give an example of how you could demonstrate the relationship between heat and a form of energy other than mechanical work.
Interpretation: The balanced equations for the given reaction statements are to be identified.
Concept introduction: The relation of the work and the heat produced is calculated by the Joule experiment. The mechanical equivalent of heat is the ratio of the heat produced from the mechanical work.
(a)
To determine: The measurements required to test the hypothesis using device of Joule’s experiment.
Answer to Problem 1DE
Solution: The measurements required to test the hypothesis using device of Joule’s experiment is stated below.
Explanation of Solution
The setup required for the experiment is,
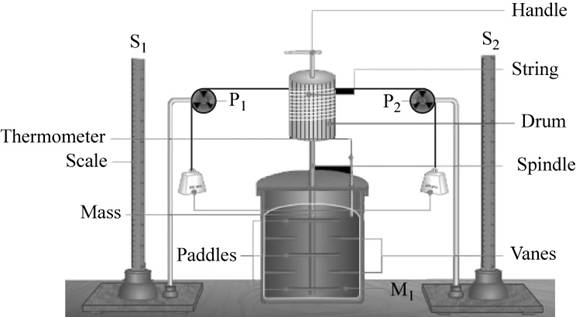
Figure 1
The relation between the work done and the heat produced is calculated in the following manner.
Using the above setup, the mechanical work is obtained by using potential energy lost by the falling mass.
Therefore, work done is equal to the potential energy of the falling mass.
Thus, the work done is calculated by the formula,
The heat that is generated in the mass of water in the calorimeter chamber is calculated by the formula,
where, water equivalent of calorimeter is the amount of water that absorbs the same amount of heat as the calorimeter to raise the temperature by one degree.
For the comparison of the work done with heat, the measurements that we need is,
- Mass of the falling object.
- Mass of water in the calorimeter.
- Height of the falling mass.
- Change in temperature.
- Water equivalent of calorimeter.
For the comparison of the work done with heat, the measurements that we need is,
- Mass of the falling object.
- Mass of water in the calorimeter.
- Height of the falling mass.
- Change in temperature.
- Water equivalent of calorimeter.
(b)
To determine: The equations used to analyze the experiment.
Answer to Problem 1DE
Solution: The equations used to analyze the experiment are stated below.
Explanation of Solution
The relation between the work done and the heat produced is to be determined.
Using the above setup, the mechanical work is obtained by using potential energy lost by the falling mass.
Therefore, work done is equal to the potential energy of the falling mass.
Thus, the work done is calculated by the formula,
The heat that is generated in the mass of water in the calorimeter chamber is calculated by the formula,
The ratio of the work done in generating heat is calculated by the formula,
Here, the constant is called as mechanical equivalent of heat.
The equations used to analyze the experiment are of mechanical work, heat and their ratio.
(c)
To determine: If the reasonable result is obtained from a single experiment.
Answer to Problem 1DE
Solution: No.
Explanation of Solution
The given experiment determines the relation of amount of work that is converted into heat energy.
This should be applicable for all sets of systems of thermodynamics.
Therefore, the same relation should be obtained in different setups of experiments.
Hence, the reasonable results are not obtained by single experiment.
The reasonable results are not obtained by single experiment.
(d)
To determine: The effect of precision of the instruments on the conclusion.
Answer to Problem 1DE
Solution: The precision in the measurement of the heat produced from work should be accurate for the perfect conclusion.
Explanation of Solution
The given experiment determines the relation of amount of work that is converted into heat energy.
The error that may occur is the loss of heat from the system. Therefore, the system should be properly isolated to measure appropriate heat.
Therefore, the precision in the measurement of the heat produced from work should be accurate for the perfect conclusion.
The precision in the measurement of the heat produced from work should be accurate for the perfect conclusion.
(e)
To determine: The modifications in the experiment that are done considering available modern amenities.
Answer to Problem 1DE
Solution: The modifications in the experiment that are done considering available modern amenities are using automated mechanical paddle stirrer and digital calorimeter.
Explanation of Solution
The mechanical work in the ancient experiment is performed by the falling masses.
Nowadays, automated mechanical paddle stirrer is available, that can be used to create mechanical work.
Also, the digital calorimeter is available that detects the change in temperature appropriately and provides an isolated system.
The modifications in the experiment that are done considering available modern amenities are using automated mechanical paddle stirrer and digital calorimeter.
(f)
To determine: The example that demonstrates the relationship between heat and a form of energy other than mechanical work.
Answer to Problem 1DE
Solution: The relationship between heat and a form of energy other than mechanical work is,
Explanation of Solution
In the above experiment, the water is stirred using a paddle with a known falling mass. The water is placed isolated in a calorimeter and a thermometer measures the temperature change in it.
The rotation in the water is obstructed by the vanes in the container. This causes the rise in temperature of water that is measured using thermometer.
The rise in temperature with the mechanical work is measured.
The relation between the work done and the heat produced is calculated in the following manner.
Using the above setup, the mechanical work is obtained by using potential energy lost by the falling mass.
Therefore, work done is equal to the potential energy of the falling mass.
Thus, the work done is calculated by the formula,
The heat that is generated in the mass of water in the calorimeter chamber is calculated by the formula,
Where, Water equivalent of calorimeter is the amount of water that absorbs the same amount of heat as the calorimeter to raise the temperature by one degree.
The ratio of the work done in generating heat is calculated by the formula,
Here, the constant is called as mechanical equivalent of heat.
Therefore, mechanical equivalent of heat is the form of energy.
The above equation is modified as,
The relationship between heat and a form of energy other than mechanical work is,
Want to see more full solutions like this?
Chapter 5 Solutions
Laboratory Experiments for Chemistry: The Central Science (14th Edition)
Additional Science Textbook Solutions
Chemistry: A Molecular Approach (4th Edition)
Microbiology: An Introduction
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Microbiology: An Introduction
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Chemistry: Structure and Properties (2nd Edition)
- Question 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forward
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





