
One of the important ideas of 
- Using this device, what measurements would you need to make to test your hypothesis?
- What equations would you use in analyzing your experiment?
- Do you think you could obtain a reasonable result from a single experiment? Why or why not?
- In what way could the precision of your instruments affect the conclusions that you make?
- List ways that you could modify the equipment to improve the data you obtain if you were performing this experiment today instead of 180 years ago.
- Give an example of how you could demonstrate the relationship between heat and a form of energy other than mechanical work.
Interpretation: The balanced equations for the given reaction statements are to be identified.
Concept introduction: The relation of the work and the heat produced is calculated by the Joule experiment. The mechanical equivalent of heat is the ratio of the heat produced from the mechanical work.
(a)
To determine: The measurements required to test the hypothesis using device of Joule’s experiment.
Answer to Problem 1DE
Solution: The measurements required to test the hypothesis using device of Joule’s experiment is stated below.
Explanation of Solution
The setup required for the experiment is,
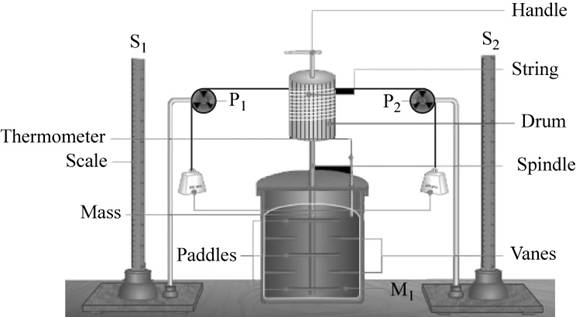
Figure 1
The relation between the work done and the heat produced is calculated in the following manner.
Using the above setup, the mechanical work is obtained by using potential energy lost by the falling mass.
Therefore, work done is equal to the potential energy of the falling mass.
Thus, the work done is calculated by the formula,
The heat that is generated in the mass of water in the calorimeter chamber is calculated by the formula,
where, water equivalent of calorimeter is the amount of water that absorbs the same amount of heat as the calorimeter to raise the temperature by one degree.
For the comparison of the work done with heat, the measurements that we need is,
- Mass of the falling object.
- Mass of water in the calorimeter.
- Height of the falling mass.
- Change in temperature.
- Water equivalent of calorimeter.
For the comparison of the work done with heat, the measurements that we need is,
- Mass of the falling object.
- Mass of water in the calorimeter.
- Height of the falling mass.
- Change in temperature.
- Water equivalent of calorimeter.
(b)
To determine: The equations used to analyze the experiment.
Answer to Problem 1DE
Solution: The equations used to analyze the experiment are stated below.
Explanation of Solution
The relation between the work done and the heat produced is to be determined.
Using the above setup, the mechanical work is obtained by using potential energy lost by the falling mass.
Therefore, work done is equal to the potential energy of the falling mass.
Thus, the work done is calculated by the formula,
The heat that is generated in the mass of water in the calorimeter chamber is calculated by the formula,
The ratio of the work done in generating heat is calculated by the formula,
Here, the constant is called as mechanical equivalent of heat.
The equations used to analyze the experiment are of mechanical work, heat and their ratio.
(c)
To determine: If the reasonable result is obtained from a single experiment.
Answer to Problem 1DE
Solution: No.
Explanation of Solution
The given experiment determines the relation of amount of work that is converted into heat energy.
This should be applicable for all sets of systems of thermodynamics.
Therefore, the same relation should be obtained in different setups of experiments.
Hence, the reasonable results are not obtained by single experiment.
The reasonable results are not obtained by single experiment.
(d)
To determine: The effect of precision of the instruments on the conclusion.
Answer to Problem 1DE
Solution: The precision in the measurement of the heat produced from work should be accurate for the perfect conclusion.
Explanation of Solution
The given experiment determines the relation of amount of work that is converted into heat energy.
The error that may occur is the loss of heat from the system. Therefore, the system should be properly isolated to measure appropriate heat.
Therefore, the precision in the measurement of the heat produced from work should be accurate for the perfect conclusion.
The precision in the measurement of the heat produced from work should be accurate for the perfect conclusion.
(e)
To determine: The modifications in the experiment that are done considering available modern amenities.
Answer to Problem 1DE
Solution: The modifications in the experiment that are done considering available modern amenities are using automated mechanical paddle stirrer and digital calorimeter.
Explanation of Solution
The mechanical work in the ancient experiment is performed by the falling masses.
Nowadays, automated mechanical paddle stirrer is available, that can be used to create mechanical work.
Also, the digital calorimeter is available that detects the change in temperature appropriately and provides an isolated system.
The modifications in the experiment that are done considering available modern amenities are using automated mechanical paddle stirrer and digital calorimeter.
(f)
To determine: The example that demonstrates the relationship between heat and a form of energy other than mechanical work.
Answer to Problem 1DE
Solution: The relationship between heat and a form of energy other than mechanical work is,
Explanation of Solution
In the above experiment, the water is stirred using a paddle with a known falling mass. The water is placed isolated in a calorimeter and a thermometer measures the temperature change in it.
The rotation in the water is obstructed by the vanes in the container. This causes the rise in temperature of water that is measured using thermometer.
The rise in temperature with the mechanical work is measured.
The relation between the work done and the heat produced is calculated in the following manner.
Using the above setup, the mechanical work is obtained by using potential energy lost by the falling mass.
Therefore, work done is equal to the potential energy of the falling mass.
Thus, the work done is calculated by the formula,
The heat that is generated in the mass of water in the calorimeter chamber is calculated by the formula,
Where, Water equivalent of calorimeter is the amount of water that absorbs the same amount of heat as the calorimeter to raise the temperature by one degree.
The ratio of the work done in generating heat is calculated by the formula,
Here, the constant is called as mechanical equivalent of heat.
Therefore, mechanical equivalent of heat is the form of energy.
The above equation is modified as,
The relationship between heat and a form of energy other than mechanical work is,
Want to see more full solutions like this?
Chapter 5 Solutions
Chemistry: The Central Science (14th Edition)
Additional Science Textbook Solutions
Chemistry: A Molecular Approach (4th Edition)
Microbiology: An Introduction
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Microbiology: An Introduction
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Chemistry: Structure and Properties (2nd Edition)
- Use the literature Ka value of the acetic acid, and the data below to answer these questions. Note: You will not use the experimental titration graphs to answer the questions that follow. Group #1: Buffer pH = 4.35 Group #2: Buffer pH = 4.70 Group #3: Buffer pH = 5.00 Group #4: Buffer pH = 5.30 Use the Henderson-Hasselbalch equation, the buffer pH provided and the literature pKa value of acetic acid to perform the following: a) calculate the ratios of [acetate]/[acetic acid] for each of the 4 groups buffer solutions above. b) using the calculated ratios, which group solution will provide the best optimal buffer (Hint: what [acetate]/[acetic acid] ratio value is expected for an optimal buffer?) c) explain your choicearrow_forwardHow would you prepare 1 liter of a 50 mM Phosphate buffer at pH 7.5 beginning with K3PO4 and 1 M HCl or 1 M NaOH? Please help and show calculations. Thank youarrow_forwardDraw the four most importantcontributing structures of the cation intermediate thatforms in the electrophilic chlorination of phenol,(C6H5OH) to form p-chlorophenol. Put a circle aroundthe best one. Can you please each step and also how you would approach a similar problem. Thank you!arrow_forward
- A 100mM lactic acid/lactate buffer was found to have a lactate to lactic acid ratio of 2 and a pH of 4.2. What is the pKa of lactic acid? Can you please help show the calculations?arrow_forwardUsing line angle formulas, draw thestructures of and name four alkanes that have total of 7carbons, one of which is tertiary.Please explain this in detail and can you also explain how to approach a similar problem like this as well?arrow_forwardUsing dashed line wedge projections drawthe indicated compounds and indicate whether thecompound you have drawn is R or S.(a) The two enantiomers of 2-chlorobutane. Can you please explain your steps and how you would approach a similar problem. Thank you!arrow_forward
- 5) There are no lone pairs shown in the structure below. Please add in all lone pairs and then give the hybridization scheme for the compound. (8) 10,11 7) 1.2.3 H 4 | 14 8) COC 12 13 H 16 15 H7 9) - 5.6 C 8 H 10) H 1). 2) 3)_ 11) 12) 13) 4)_ 14) 5) 15) 16) 6)arrow_forwardThe sum of the numbers in the name of isA. 11; B. 13; C. 10; D. 12; E. none of the other answers iscorrect. I believe the awnser should be E to this problem but the solution to this problem is D 12. I'm honestly unsure how that's the solution. If you can please explain the steps to this type of problem and how to approach a problem like this it would be greatly appreciated!arrow_forwardConsider the following data for phosphorus: g atomic mass 30.974 mol electronegativity 2.19 kJ electron affinity 72. mol kJ ionization energy 1011.8 mol kJ heat of fusion 0.64 mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? 2+ + (1) P (g) + e → P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): Does the following reaction absorb or release energy? 00 release absorb Can't be decided with the data given. yes no ☐ kJ/mol (²) P* (8) + + + e →>> P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (2) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (2): ☐ release absorb Can't be decided with the data given. yes no kJ/mol аarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





