
Concept explainers
One Tough Bug The genus Ferroplasma consists of a few species of acid-loving archaea. One species, Ferroplasma acidarmanus, was discovered in one of the most contaminated sites in the United States: Iron Mountain Mine in California. F. acidarmamus is the main constituent of .slime streamers (a type of biofilm) growing in water draining from this abandoned copper mine (right). The water is hot (about 40°C, or 104°F), heavily laden with arsenic and other toxic metals, and has a pH of zero.
F. acidarmanus cells have an ancient energy-harvesting pathway that uses electrons pulled from iron-sulfur compounds in minerals such as pyrite. Removing electrons from these compounds dissolves the minerals, so groundwater in the mine ends up with extremely high concentrations of solutes, including metal ions such as copper, zinc, cadmium, and arsenic. The pathway also produces .sulfuric acid, which lowers the pH of the water around the cell to zero.
F. acidarmanus cells keep their internal pH at a cozy 5.0 despite Living in an environment similar to hot battery acid. However, most of the cell’s enzymes function best al much lower pH (FIGURE 5.13). Thus, researchers think F. acidarmanus may have an unknown type of internal compartment that keeps their enzymes in a highly acidic environmental.

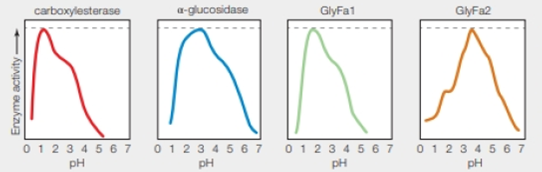
FIGURE 5.13 pH anomaly of Ferroplasma acidarmanus.
Left graphs showing pH activity profile of four enzymes isolated from Ferraplasma acidarmanus Researchers had expected all of these enzymes to function best at the calls’ cytoplasmic pH (5.0).
What do the dashed lines in the graphs signify?
To determine: What do the dashed lines in the graphs signify.
Concept introduction: Enzymes are the protein molecules that enhance the rate of the reactions without being changed by them. It acts as a biocatalyst. Each enzyme works the best within a certain range of environmental conditions that include temperature, pH, and salt concentration.
Explanation of Solution
The isolated bacteria Ferroplasma acidarmanus grow well at acidic environment (pH zero) of copper mine where the water temperature is about 40 ºC and heavily laden with arsenic and other toxic metals.
The F. acidarmanus has an ancient energy harvesting pathway. It uses the electrons from iron-sulfur compounds in the mineral such as pyrite. Removing electrons from these compounds dissolve the minerals that lead to the high concentration of metal ions such as copper, zinc, cadmium, and arsenic in groundwater. This also produces sulfuric acid which lowers the pH of the water around the cells to zero.
Despite the surrounding environment the internal pH of the bacteria is 5.0. However, most of the enzymes in bacteria functions best at much lower pH. This causes a thinking of presence of the unknown type of internal environment in the bacteria that keeps their enzymes in a highly acidic environment.
Refer to Fig. 5.13 “pH anomaly of Ferroplasma acidarmanus”, there are four different enzymes carboxylesterase, α-glucosidase, GlyFa1, and GlyFa2 which works best around pH 1.5, 3, 1.8, and 3.8 respectively. The dashed line shows the optimum activity for each enzyme.
The dashed line indicates the optimum enzyme activity for each enzyme.
Want to see more full solutions like this?
Chapter 5 Solutions
Biology: The Unity and Diversity of Life (Looseleaf)
Additional Science Textbook Solutions
Genetics: From Genes to Genomes
Laboratory Manual For Human Anatomy & Physiology
Microbiology Fundamentals: A Clinical Approach
Human Physiology: An Integrated Approach (8th Edition)
- What is a gain of function mutation?arrow_forwardMolecular Biology Question: Please help. Thank you Is Southern hybridization's purpose detecting specific nucleotide sequences? How so?arrow_forwardUse the following information to answer the question(s) below. Martin Wikelski and L. Michael Romero (Body size, performance and fitness in Galápagos marine iguanas, Integrative and Comparative Biology 43 [2003]:376-86) measured the snout-to-vent (anus) length of Galápagos marine iguanas and observed the percent survival of different-sized animals, all of the same age. The graph shows the log snout-vent length (SVL, a measure of overall body size) plotted against the percent survival of these different size classes for males and females. Survival (%) 100- 80- 60- 40- 20- 0+ 1.9 T 2 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Log SVL (mm) 19) Examine the figure above. What type of selection for body size appears to be occurring in these marine iguanas? A) directional selection B) stabilizing selection C) disruptive selection D) You cannot determine the type of selection from the above information. 3arrow_forward
- 24) Use the following information to answer the question below. Researchers studying a small milkweed population note that some plants produce a toxin and other plants do not. They identify the gene responsible for toxin production. The dominant allele (T) codes for an enzyme that makes the toxin, and the recessive allele (t) codes for a nonfunctional enzyme that cannot produce the toxin. Heterozygotes produce an intermediate amount of toxin. The genotypes of all individuals in the population are determined (see table) and used to determine the actual allele frequencies in the population. TT 0.49 Tt 0.42 tt 0.09 Refer to the table above. Is this population in Hardy-Weinberg equilibrium? A) Yes. C) No; there are more homozygotes than expected. B) No; there are more heterozygotes than expected. D) It is impossible to tell.arrow_forward30) A B CDEFG Refer to the accompanying figure. Which of the following forms a monophyletic group? A) A, B, C, and D B) C and D C) D, E, and F D) E, F, and Garrow_forwardMolecular Biology Question. Please help with step solution and explanation. Thank you: The Polymerase Chain Reaction (PCR) reaction consists of three steps denaturation, hybridization, and elongation. Please describe what occurs in the annealing step of the PCR reaction. (I think annealing step is hybridization). What are the other two steps of PCR, and what are their functions? Next, suppose the Tm for the two primers being used are 54C for Primer A and 67C for Primer B. Regarding annealing step temperature, I have the following choices for the temperature used during the annealing step:(a) 43C (b) 49C (c) 62C (d) 73C Which temperature/temperatures should I choose? What is the corresponding correct explanation, and why would I not use the other temperatures? Have a good day!arrow_forward
- Using the data provided on the mean body mass and horn size of 4-year-old male sheep, draw a scatterplot graph to examine how body mass and horn size changed over time.arrow_forwardPlease write a 500-word report about the intake of saturated fat, sodium, alcoholic beverages, or added sugar in America. Choose ONE of these and write about what is recommended by the Dietary Guidelines for Americans (guideline #4) and why Americans exceed the intake of that nutrient. Explain what we could do as a society and/or individuals to reduce our intake of your chosen nutrient.arrow_forwardWrite a 500-word report indicating how you can change the quantity or quality of TWO nutrients where your intake was LOWER than what is recommended by the Dietary Guidelines for Americans and/or the DRIs. Indicate how the lack of the nutrient may affect your health. For full credit, all of the following points must be addressed and elaborated on in more detail for each nutrient: The name of the nutrient At least 2 main functions of the nutrient (example: “Vitamin D regulates calcium levels in the blood and calcification of bones.”) Your percent intake compared to the RDA/DRI (example “I consumed 50% of the RDA for vitamin D”) Indicate why your intake was below the recommendations (example: “I only had one serving of dairy products and that was why I was below the recommendations for vitamin D”) How would you change your dietary pattern to meet the recommendations? – be sure to list specific foods (example: “I would add a yogurt and a glass of milk to each day in order to increase my…arrow_forward
- Why are nutrient absorption and dosage levels important when taking multivitamins and vitamin and mineral supplements?arrow_forwardI'm struggling with this topic and would really appreciate your help. I need to hand-draw a diagram and explain the process of sexual differentiation in humans, including structures, hormones, enzymes, and other details. Could you also make sure to include these terms in the explanation? . Gonads . Wolffian ducts • Müllerian ducts . ⚫ Testes . Testosterone • Anti-Müllerian Hormone (AMH) . Epididymis • Vas deferens ⚫ Seminal vesicles ⚫ 5-alpha reductase ⚫ DHT - Penis . Scrotum . Ovaries • Uterus ⚫ Fallopian tubes - Vagina - Clitoris . Labia Thank you so much for your help!arrow_forwardRequisition Exercise A phlebotomist goes to a patient’s room with the following requisition. Hometown Hospital USA 125 Goodcare Avenue Small Town, USAarrow_forward
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax





