
Concept explainers
(a)
Interpretation:
The second resonance structure of the following ion should be drawn:
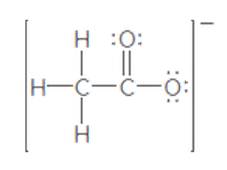
Concept Introduction:
When more than one Lewis structure can be drawn for a molecule or ion, then it is said to have resonance.
It is generally the delocalization of electrons (bonds) over three or more atoms in a molecule for which one simple Lewis structure cannot depicts its correct structure.
(b)
Interpretation:
The second resonance structure of the following ion should be drawn:
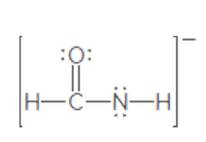
Concept Introduction:
When more than one Lewis structure can be drawn for a molecule or ion, then it is said to have resonance.
It is generally the delocalization of electrons (bonds) over three or more atoms in a molecule for which one simple Lewis structure cannot depicts its correct structure.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
General, Organic, and Biological Chemistry - 4th edition
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning





