
Concept explainers
(a)
Interpretation:
Given compound has to be named.
Concept Introduction:
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent.
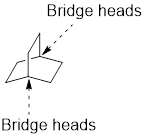
Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(b)
Interpretation:
Given compound has to be named.
Concept Introduction:
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] (each number represent number of carbon atoms) and placed in the middle of the parent.
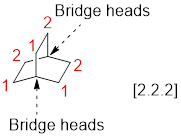
Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(c)
Interpretation:
Given compound has to be named.
Concept Introduction:
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent.

Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(d)
Interpretation:
Given compound has to be named.
Concept Introduction:
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent.

Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(e)
Interpretation:
Given compound has to be named.
Concept Introduction:
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent.

Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
Trending nowThis is a popular solution!

Chapter 4 Solutions
Organic Chemistry
- Draw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forwardExplanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forwardShow the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forward
- Soap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- Provide the mechanism for this transformation: *see imagearrow_forwardAssign all the signals individually (please assign the red, green and blue)arrow_forwardThe two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





