
Organic Chemistry
3rd Edition
ISBN: 9781119338352
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Chapter 4, Problem 44PP
(a)
Interpretation Introduction
Interpretation:
The compound is needed to found which has more heat of combustion value between the given set of compounds.
Concept introduction:
- Heat of combustion: heat of combustion is the numeric value, which represents the energy required to burn a hydrocarbon completely in presence of oxygen. For more stable molecule heat of combustion value will be high when compare with least stable molecule.
- Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:
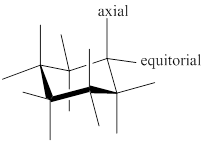
- Equatorial position is more stable than axial position; therefore a compound with equatorial substituents has high heat of combustion value than a compound with axial substituents.
(b)
Interpretation Introduction
Interpretation:
The compound is needed to found which has more heat of combustion value between the given set of compounds.
Concept introduction:
- Heat of combustion: heat of combustion is the numeric value, which represents the energy required to burn a hydrocarbon completely in presence of oxygen. For more stable molecule heat of combustion value will be high when compare with least stable molecule.
- Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

- Equatorial position is more stable than axial position; therefore a compound with equatorial substituents has high heat of combustion value than a compound with axial substituents.
(c)
Interpretation Introduction
Interpretation:
The compound is needed to found which has more heat of combustion value between the given set of compounds.
Concept introduction:
- Heat of combustion: heat of combustion is the numeric value, which represents the energy required to burn a hydrocarbon completely in presence of oxygen. For more stable molecule heat of combustion value will be high when compare with least stable molecule.
- Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

- Equatorial position is more stable than axial position; therefore a compound with equatorial substituents has high heat of combustion value than a compound with axial substituents.
(d)
Interpretation Introduction
Interpretation:
The compound is needed to found which has more heat of combustion value between the given set of compounds.
Concept introduction:
- Heat of combustion: heat of combustion is the numeric value, which represents the energy required to burn a hydrocarbon completely in presence of oxygen. For more stable molecule heat of combustion value will be high when compare with least stable molecule.
- Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

- Equatorial position is more stable than axial position; therefore a compound with equatorial substituents has high heat of combustion value than a compound with axial substituents.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
julietteyep@gmail.com
X
YSCU Grades for Juliette L Turner: Orc X
199
A ALEKS - Juliette Turner - Modul X A ALEKS - Juliette Turner - Modul x G butane newman projection - Gox
+
www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBxzaplnN4HsoQggFsejpgqKoyrQrB2dKVAN-BcZvcye0LYa6eXZ8d4vVr8Nc1GZqko5mtw-d1MkNcNzzwZsLf2Tu9_V817y?10Bw7QYjlb
il
Scribbr citation APA
SCU email
Student Portal | Main
Ryker-Learning
WCU-PHARM D MySCU YSCU Canvas- SCU
Module 4: Homework (Ch 9-10)
Question 28 of 30 (1 point) | Question Attempt: 1 of Unlimited
H₂SO
heat
OH
The mechanism of this reaction involves two carbocation intermediates, A and B.
Part 1 of 2
KHSO
4
rearrangement
A
heat
B
H₂O
2
OH
Draw the structure of A.
Check
Search
#t
m
Save For Later
Juliet
Submit Assignm
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access
The electrons flow from the electron-rich atoms of the nucleophile to the electrons poor atoms of the alkyl halide. Identify the electron rich in the nucleophile. Enter the element symbol only, do not include any changes.
Hello, I am doing a court case analysis in my Analytical Chemistry course.
The case is about a dog napping and my role is prosecution of the defendant.
I am tasked in the Area of Expertise in Neutron Activation and Isotopic Analysis.
Attached is the following case study reading of my area of expertise!
The landscaping stone was not particularly distinctive in its decoration but matched both the color and pattern of the Fluential’s landscaping stone as well as the stone in the back of the recovered vehicle. Further analysis of the stone was done using a technique called instrumental neutron activation analysis. (Proceed to Neutron Activation data)
Photo Notes: Landscaping stone recovered in vehicle. Stone at Fluential’s home is similar inappearance.
Finally, the white paint on the brick was analyzed using stable isotope analysis. The brick recovered at the scene had smeared white paint on it. A couple of pieces of brick in the back of the car had white paint on them. They…
Chapter 4 Solutions
Organic Chemistry
Ch. 4.2 - Prob. 1LTSCh. 4.2 - Prob. 1PTSCh. 4.2 - Prob. 2PTSCh. 4.2 - Prob. 3ATSCh. 4.2 - Prob. 2LTSCh. 4.2 - Prob. 4PTSCh. 4.2 - Prob. 5ATSCh. 4.2 - Prob. 3LTSCh. 4.2 - Prob. 6PTSCh. 4.2 - Prob. 7ATS
Ch. 4.2 - Prob. 4LTSCh. 4.2 - Prob. 8PTSCh. 4.2 - Prob. 9PTSCh. 4.2 - Prob. 10ATSCh. 4.2 - Prob. 5LTSCh. 4.2 - Prob. 11PTSCh. 4.2 - Prob. 12PTSCh. 4.2 - Prob. 13ATSCh. 4.3 - Prob. 6LTSCh. 4.3 - Prob. 14PTSCh. 4.3 - Prob. 15ATSCh. 4.6 - Prob. 7LTSCh. 4.6 - Prob. 16PTSCh. 4.6 - Prob. 17ATSCh. 4.7 - Prob. 18CCCh. 4.8 - Prob. 8LTSCh. 4.8 - Prob. 19PTSCh. 4.8 - Prob. 20ATSCh. 4.11 - Prob. 9LTSCh. 4.11 - Prob. 21PTSCh. 4.11 - Prob. 22ATSCh. 4.11 - Prob. 10LTSCh. 4.11 - Prob. 23PTSCh. 4.11 - Prob. 24ATSCh. 4.12 - Prob. 11LTSCh. 4.12 - Prob. 25PTSCh. 4.12 - Prob. 26ATSCh. 4.12 - Prob. 27CCCh. 4.13 - Prob. 12LTSCh. 4.13 - Prob. 28PTSCh. 4.13 - Prob. 29ATSCh. 4.13 - Prob. 13LTSCh. 4.13 - Prob. 30PTSCh. 4.13 - Prob. 31ATSCh. 4.13 - Prob. 32ATSCh. 4.14 - Prob. 33CCCh. 4.14 - Prob. 34CCCh. 4.14 - Prob. 35CCCh. 4 - Prob. 36PPCh. 4 - Prob. 37PPCh. 4 - Prob. 38PPCh. 4 - Prob. 39PPCh. 4 - Prob. 40PPCh. 4 - Prob. 41PPCh. 4 - Prob. 42PPCh. 4 - Prob. 43PPCh. 4 - Prob. 44PPCh. 4 - Prob. 45PPCh. 4 - Prob. 46PPCh. 4 - Prob. 47PPCh. 4 - Prob. 48PPCh. 4 - Prob. 49PPCh. 4 - Prob. 50PPCh. 4 - Prob. 51PPCh. 4 - Prob. 52PPCh. 4 - Prob. 53PPCh. 4 - Prob. 54PPCh. 4 - Prob. 55PPCh. 4 - Prob. 56PPCh. 4 - Prob. 57PPCh. 4 - Prob. 58PPCh. 4 - Prob. 59PPCh. 4 - Prob. 60PPCh. 4 - Prob. 61IPCh. 4 - Prob. 62IPCh. 4 - Prob. 64IPCh. 4 - Prob. 65IPCh. 4 - Prob. 66IPCh. 4 - Prob. 67IPCh. 4 - Prob. 68IPCh. 4 - Prob. 69IPCh. 4 - Prob. 70IPCh. 4 -
All of the following are representations of...Ch. 4 - Prob. 72IPCh. 4 -
Which of the following is expected to have the...Ch. 4 - Prob. 74IPCh. 4 - Prob. 75CPCh. 4 -
The all-trans-1,2,3,4,5,6-hexaethylcyclohexane...Ch. 4 -
Compounds 1 and 2 were prepared, and the...
Knowledge Booster
Similar questions
- What is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forward
- What is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forward
- What would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY