
Concept explainers
Using the cyclohexane with the C’s numbered as shown, draw a chair form that fits each
description.
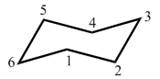
a. The ring has an axial
b. The ring has an equatorial
c. The ring has equatorial
Trending nowThis is a popular solution!

Chapter 4 Solutions
Study Guide/solutions Manual For Organic Chemistry
Additional Science Textbook Solutions
Biology: Life on Earth with Physiology (11th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Physical Science
Organic Chemistry
- A DEPT NMR spectrum is shown for a molecule with the molecular formula of C5H12O. Draw the structure that best fits this data. 200 180 160 140 120 100 一盆 00 40 8- 20 ppm 0 Qarrow_forwardDon't used hand raitingarrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. H. +N=C H H H Cl: Click and drag to start drawing a structure. : ? g B S olo Ar B Karrow_forward
- Don't used hand raitingarrow_forwardS Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H H = HIN: H C. :0 H /\ H H Click and drag to start drawing a structure. ×arrow_forwardPlease help me figure out these calculation and what should be plotted. These are notes for my chemistry class.arrow_forward
- Nonearrow_forwardNonearrow_forwardPart II. two unbranched ketone have molecular formulla (C8H100). El-ms showed that both of them have a molecular ion peak at m/2 =128. However ketone (A) has a fragment peak at m/2 = 99 and 72 while ketone (B) snowed a fragment peak at m/2 = 113 and 58. 9) Propose the most plausible structures for both ketones b) Explain how you arrived at your conclusion by drawing the Structures of the distinguishing fragments for each ketone, including their fragmentation mechanisms.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
