
Biological Science (6th Edition)
6th Edition
ISBN: 9780321976499
Author: Scott Freeman, Kim Quillin, Lizabeth Allison, Michael Black, Emily Taylor, Greg Podgorski, Jeff Carmichael
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 40, Problem 14PIAT
Summary Introduction
To review:
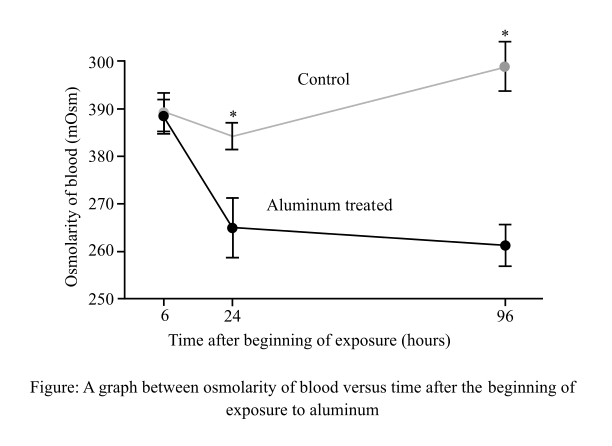
The results related to the activity of Na+/K+-ATPase (sodium/potassium adenosine triphosphatase) in the gills of the fish exposed to aluminum and its comparison with the control fish.
Introduction:
The Na+/K+- ATPase is the enzyme, which works as a pump, and is found in the plasma membrane of the cell. Its function is to allow the movement of sodium ions out of the cell and potassium ions into the cell. Scientists exposed freshwater bony fish (Prochilodus lineatus) exposed to water with high aluminum level and another set of fish that was exposed to water with normal aluminum levels (control). The results of the data are shown in graph below:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Based on your results from the Mannitol Salt Agar (MSA) media, which of your bacteria were mannitol fermenters and which were not mannitol fermenters?
help tutor please
Q8. A researcher wants to study the effectiveness of a pill intended to reduce stomach heartburn in pregnant
women. The researcher chooses randomly 400 women to participate in this experiment for 9 months of their
pregnancy period. They all need to have the same diet. The researcher designs two groups of 200 participants:
One group take the real medication intended to reduce heartburn, while the other group take placebo
medication. In this study what are:
Independent variable:
Dependent variable:
Control variable:
Experimental group: "
Control group:
If the participants do not know who is consuming the real pills and who is consuming the sugar pills.
This study is
It happens that 40% of the participants do not find the treatment helpful and drop out after 6 months.
The researcher throws out the data from subjects that drop out. What type of bias is there in this study?
If the company who makes the medication funds this research, what type of bias might exist in this
research work?
Chapter 40 Solutions
Biological Science (6th Edition)
Ch. 40 - 1. Which of the following statements is true of...Ch. 40 - Prob. 2TYKCh. 40 - 3. What effect does antidiuretic hormone (ADH)...Ch. 40 - Fill in the blank: In Gila monsters, the organ in...Ch. 40 - Prob. 5TYUCh. 40 - Prob. 6TYUCh. 40 - Prob. 7TYUCh. 40 - 8. Scientists have noted that marine invertebrates...Ch. 40 - Prob. 9TYPSSCh. 40 - Prob. 10TYPSS
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- What is behavioral adaptarrow_forward22. Which of the following mutant proteins is expected to have a dominant negative effect when over- expressed in normal cells? a. mutant PI3-kinase that lacks the SH2 domain but retains the kinase function b. mutant Grb2 protein that cannot bind to RTK c. mutant RTK that lacks the extracellular domain d. mutant PDK that has the PH domain but lost the kinase function e. all of the abovearrow_forwardWhat is the label ?arrow_forward
- Can you described the image? Can you explain the question as well their answer and how to get to an answer to an problem like this?arrow_forwardglg 112 mid unit assignment Identifying melting processesarrow_forwardGive only the mode of inheritance consistent with all three pedigrees and only two reasons that support this, nothing more, (it shouldn't take too long)arrow_forward
- Oarrow_forwardDescribe the principle of homeostasis.arrow_forwardExplain how the hormones of the glands listed below travel around the body to target organs and tissues : Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning



Photosynthesis & Respiration | Reactions | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=3XIyweZg6Sw;License: Standard YouTube License, CC-BY