
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4, Problem 61P
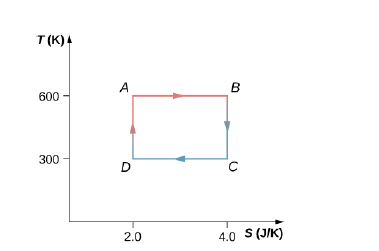
The Carnot cycle is represented by the temperature-entropy diagram shown below. (a) How much heat is absorbed per cycle at the high-temperature reservoir? (b) How much heat is exhausted per cycle at the low-temperature reservoir? (c) How much work is done per cycle by the engine? (d) What is the efficiency of the engine?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
8.
With the aid of a diagram draw the following electric circuit and use the resistor as the load,
(a) Closed circuit
(b) Open circuit
Lab 8 Part 3 PHET Wave Interface simulation.
I am having trouble with this part of the lab.
Mick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?
Chapter 4 Solutions
University Physics Volume 2
Ch. 4 - Check Your Understanding What is the efficiency of...Ch. 4 - Check your Understanding Show that QhQh=QcQc for...Ch. 4 - Check Your Understanding A Carnot engine operates...Ch. 4 - Check Your Understanding A Carnot refrigerator...Ch. 4 - Check Your Understanding In Example 4.7, the...Ch. 4 - Check Your Understanding A quantity of heat Q is...Ch. 4 - Check Your Understanding A 50-g copper piece at a...Ch. 4 - State an example of a process that occurs in...Ch. 4 - Explain in practical terms why efficiency is...Ch. 4 - If the refrigerator door is left what happens to...
Ch. 4 - Is it possible for the efficiency of a reversible...Ch. 4 - In the text, we showed that if the Clausius...Ch. 4 - Why don't we operate ocean liners by extracting...Ch. 4 - Discuss the practical advantages and disadvantages...Ch. 4 - The energy output of a heat pump is greater than...Ch. 4 - Speculate as to why nuclear power plants are less...Ch. 4 - An ideal gas goes from state (pi,vi,) to state...Ch. 4 - To increase the efficiency of a Carnot engine,...Ch. 4 - How could you design a Carnot engine with 100%...Ch. 4 - What type of processes occur in a Carnot cycle?Ch. 4 - Does the entropy increase for a Carnot engine for...Ch. 4 - Is it possible for a system to have an entropy...Ch. 4 - Are the entropy changes of the system in the...Ch. 4 - Discuss the entropy changes in the systems of...Ch. 4 - A tank contains 111.0 g chlorine gas l2), which is...Ch. 4 - A mole of ideal monatomic gas at 0 and 1.00 atm...Ch. 4 - A mole of an ideal gas at pressure 4.00 atm and...Ch. 4 - After a free expansion to quadruple its volume, a...Ch. 4 - An engine is found to have an efficiency of 0.40....Ch. 4 - In performing 100.0 J of work, an engine...Ch. 4 - An engine with an efficiency of 0.30 absorbs 500 J...Ch. 4 - It is found that an engine discharges 100.0 J...Ch. 4 - The temperature of the cold reservoir of the...Ch. 4 - An engine absorbs three times as much heat as it...Ch. 4 - A coal power plant consumes 100,000 kg of coal per...Ch. 4 - A refrigerator has a coefficient of performance of...Ch. 4 - During one cycle, a refrigerator removes 500 J...Ch. 4 - If a refrigerator discards 80 J of heat per cycle...Ch. 4 - A refrigerator has a coefficient of performance of...Ch. 4 - The temperature of the cold and hot reservoirs...Ch. 4 - Suppose a Carnot refrigerator operates between Tc...Ch. 4 - A Carnot engine operates between reservoirs at 600...Ch. 4 - A 500-W motor operates a Carnot refrigerator...Ch. 4 - Sketch a Carnot cycle on a temperature-volume...Ch. 4 - A Carnot heat pump operates between 0 and 20 ....Ch. 4 - An engine between heat reservoirs at 20 and 200 ...Ch. 4 - Suppose a Carnot engine can be operated between...Ch. 4 - A Carnot engine is used to measure the temperature...Ch. 4 - What is the minimum work required of a...Ch. 4 - Two hundred joules of heat are removed from a heat...Ch. 4 - In an isothermal reversible expansion at 27 , an...Ch. 4 - An ideal gas at 300 K is compressed isothermally...Ch. 4 - What is the entropy change of 10 g of steam at 100...Ch. 4 - A metal is used to conduct heat between two...Ch. 4 - For the Carnot cycle of Figure 4.12, what is the...Ch. 4 - A 5.0-kg piece of lead at a temperature of 600 is...Ch. 4 - One mole of an ideal gas doubles its volume in a...Ch. 4 - One mole of an ideal monatomic gas is confined to...Ch. 4 - (a) A 5.0-kg rock at a temperature of 20 is...Ch. 4 - A copper rod of cross-sectional area 5.0 cm2 and...Ch. 4 - Fifty grams of water at 20 is heated until it...Ch. 4 - Fifty grams of water at 0 are changed into vapor...Ch. 4 - In an isochoric process, heat is added to 10 mol...Ch. 4 - Two hundred grams of water at 0 is brought into...Ch. 4 - Suppose that the temperature of the water in the...Ch. 4 - Two hundred grams of water at 0 is brought into...Ch. 4 - (a) Ten grams of H2O stats as ice at 0 . The ice...Ch. 4 - The Carnot cycle is represented by the...Ch. 4 - A Carnot engine operating between heat reservoirs...Ch. 4 - A monoatomic ideal gas (n moles) goes through a...Ch. 4 - A Carnot engine has an efficiency of 0.60. When...Ch. 4 - A Carnot engine performs 100 J of work while...Ch. 4 - A Carnot refrigerator exhausts heat to the air,...Ch. 4 - A 300-W heat pump operates between the ground,...Ch. 4 - An engineer must design a refrigerator that does...Ch. 4 - A Carnot engine employs 1.5 mol of nitrogen gas as...Ch. 4 - A 5.0-kg wood block starts with an initial speed...Ch. 4 - A system consisting of 20.0 mol of a monoatomic...Ch. 4 - A glass beaker of mass 400 g contains 500 g of...Ch. 4 - A Carnot engine operates between 550 and 20 ...Ch. 4 - An ideal gas at temperature T is stored in the...Ch. 4 - A 0.50-kg piece of aluminum at 250 is dropped...Ch. 4 - Suppose 20 g of ice at 0 is added to 300 g of...Ch. 4 - A heat engine operates between two temperatures...Ch. 4 - A thermal engine produces 4 MJ of electrical...Ch. 4 - A coal power plant consumes 100,000 kg of coal per...Ch. 4 - A Carnot engine operates in a Carnot cycle between...Ch. 4 - A Carnot engine working between two heat baths of...Ch. 4 - A Carnot cycle working between 100 and 30 is...Ch. 4 - (a) infinitesimal amount of heat is added...Ch. 4 - Using the result of the preceding problem, show...Ch. 4 - With the help of the two preceding problems, show...Ch. 4 - A cylinder contains 500 g of helium at 120 atm and...Ch. 4 - A diatomic ideal gas is brought from an initial...Ch. 4 - The gasoline internal combustion engine operates...Ch. 4 - An ideal diesel cycle is shown below. This cycle...Ch. 4 - Consider an ideal gas Joule cycle, also called the...Ch. 4 - Derive a formula for the coefficient of...Ch. 4 - Two moles of nitrogen gas, with =7/5 for ideal...Ch. 4 - A Carnot refrigerator, working between 0 and 30 ...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
2. List the subdivisions of the thoracic and abdominopelvic cavities.
Human Anatomy & Physiology (2nd Edition)
Match the following examples of mutagens. Column A Column B ___a. A mutagen that is incorporated into DNA in pl...
Microbiology: An Introduction
How do food chains and food webs differ? Which is the more accurate representation of feeding relationships in ...
Biology: Life on Earth (11th Edition)
23. A dolphin emits ultrasound at 100 kHz and uses the timing of reflections to determine the position of objec...
College Physics: A Strategic Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- No chatgpt pls will upvotearrow_forwardYou are standing a distance x = 1.75 m away from this mirror. The object you are looking at is y = 0.29 m from the mirror. The angle of incidence is θ = 30°. What is the exact distance from you to the image?arrow_forwardFor each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank youarrow_forward
- A planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).arrow_forwardWhat are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V) ammeter I =arrow_forwardsimple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forward
- A circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forwardAn L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY