
Concept explainers
Read Appendix B on naming bicyclic compounds. Then give IUPAC name for each of the following compounds.
a. 
b. 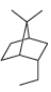
c. 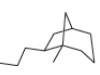
d. 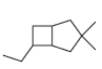
(a)
Interpretation: The IUPAC name for the given bicyclic compound is to be stated.
Concept introduction: The root name of bicyclic compounds depends upon the number of carbon atoms present in the rings. The naming of bicyclic compounds is done as,
(1) The total number of carbon atoms in the molecule is to be counted. It represents the root name.
(2) The number of carbon atoms between the bridgeheads is to be counted and placed in square brackets in descending order.
(3) The word bicyclo is placed at the beginning of the name.
The general name such compounds is represented as
Answer to Problem 4.75P
The IUPAC name for the given bicyclic compound is
Explanation of Solution
The given bicyclic compound is shown below.
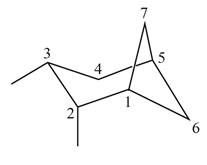
Figure 1
The given compound contains seven carbon atoms in the ring which are single bonded to each other. Therefore, the root name is heptane. In this case, there are
The IUPAC name for the given bicyclic compound is
(b)
Interpretation: The IUPAC name for the given bicyclic compound is to be stated.
Concept introduction: The root name of bicyclic compounds depends upon the number of carbon atoms present in the rings. The naming of bicyclic compounds is done as,
(1) The total number of carbon atoms in the molecule is to be counted. It represents the root name.
(2) The number of carbon atoms between the bridgeheads is to be counted and placed in square brackets in descending order.
(3) The word bicyclo is placed at the beginning of the name.
The general name such compounds is represented as
Answer to Problem 4.75P
The IUPAC name for the given bicyclic compound is
Explanation of Solution
The given bicyclic compound is shown below.
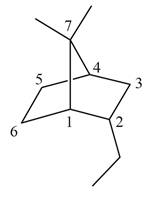
Figure 2
The given compound contains seven carbon atoms in the ring which are single bonded to each other. Therefore, the root name is heptane. In this case, there are
The IUPAC name for the given bicyclic compound is
(c)
Interpretation: The IUPAC name for the given bicyclic compound is to be stated.
Concept introduction: The root name of bicyclic compounds depends upon the number of carbon atoms present in the rings. The naming of bicyclic compounds is done as,
(1) The total number of carbon atoms in the molecule is to be counted. It represents the root name.
(2) The number of carbon atoms between the bridgeheads is to be counted and placed in square brackets in descending order.
(3) The word bicyclo is placed at the beginning of the name.
The general name such compounds is represented as
Answer to Problem 4.75P
The IUPAC name for the given bicyclic compound is
Explanation of Solution
The given bicyclic compound is shown below.
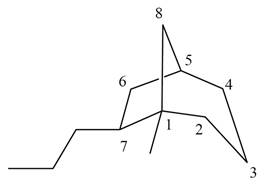
Figure 3
The given compound contains eight carbon atoms in the ring which are single bonded to each other. Therefore, the root name is octane. In this case, there are
The IUPAC name for the given bicyclic compound is
(d)
Interpretation: The IUPAC name for the given bicyclic compound is to be stated.
Concept introduction: The root name of bicyclic compounds depends upon the number of carbon atoms present in the rings. The naming of bicyclic compounds is done as,
(1) The total number of carbon atoms in the molecule is to be counted. It represents the root name.
(2) The number of carbon atoms between the bridgeheads is to be counted and placed in square brackets in descending order.
(3) The word bicyclo is placed at the beginning of the name.
The general name such compounds is represented as
Answer to Problem 4.75P
The IUPAC name for the given bicyclic compound is
Explanation of Solution
The given bicyclic compound is shown below.
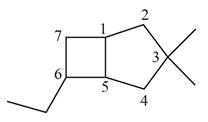
Figure 4
The given compound contains seven carbon atoms in the ring which are single bonded to each other. Therefore, the root name is heptane. In this case, there are
The IUPAC name for the given bicyclic compound is
Want to see more full solutions like this?
Chapter 4 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Physics of Everyday Phenomena
Campbell Biology: Concepts & Connections (9th Edition)
General, Organic, and Biological Chemistry - 4th edition
Laboratory Manual For Human Anatomy & Physiology
Biology: Concepts and Investigations
- Complete the following equations please hand written pleasearrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 3+ 3Cu²+ (aq) +2Al(s) → 3 Cu(s)+2A1³* (aq) 2+ Suppose the cell is prepared with 5.29 M Cu in one half-cell and 2.49 M A1³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. x10 μ ☑ 00. 18 Ar Иarrow_forwardPlease help me solve this homework problemarrow_forward
- Please help me answer this homework questionarrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3+ H2(g)+2OH¯ (aq) + 2Fe³+ (aq) → 2H₂O (1)+2Fe²+ (aq) 0 kJ x10 Х ? olo 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 184.2 mL of a 0.7800M solution of dimethylamine ((CH3) NH with a 0.3000M solution of HClO4. The pK₁ of dimethylamine is 3.27. Calculate the pH of the base solution after the chemist has added 424.1 mL of the HClO solution to it. 2 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO 4 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ ? 000 18 Ar 1 Barrow_forward
- Using the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq) + 2+ 2+ 3+ Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. ☐ x10 μ Х 5 ? 000 日。arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq) kJ - ☐ x10 x10 olo 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid b An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ 5arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning




