
Concept explainers
(a)
Interpretation:
The chemical formula of red compound in the following periodic table is to be identified.
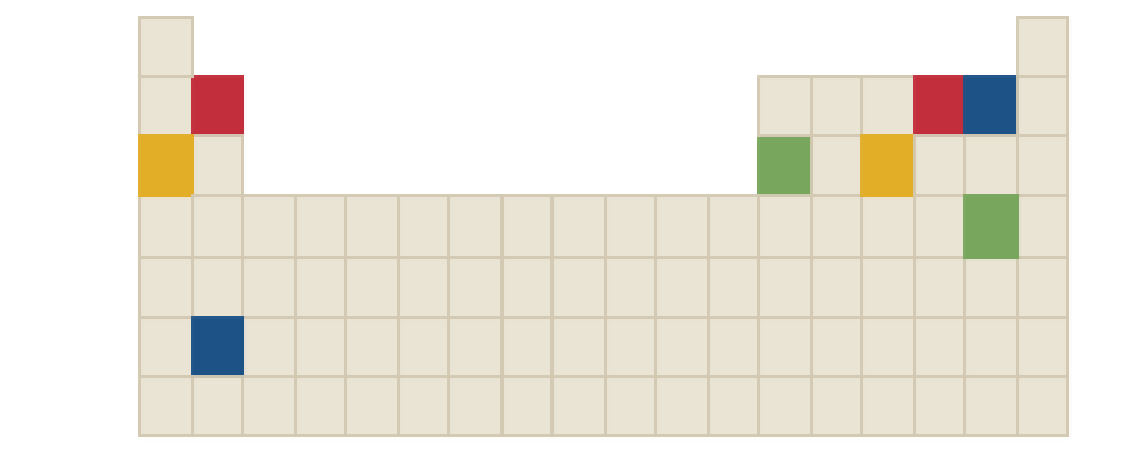
Concept Introduction:
Ions are formed by the loss or gain of electrons. The formation of ion requires the presence of two elements, these two elements are: one is metal atom and another one is non-metal atom. Metal atom loses electron and non-metal atom accepts electron.
The ratio in which positive and negative ions combine is the ratio which achieves charge neutrality for the resulting compound.
There three rules to remember while writing the chemical formulas. They are as follows:
- First write the symbol for positive ion.
- The charges of the ions are not shown in the formula.
- The numbers in the formula give the combining ratio for the ions.
(b)
Interpretation:
The chemical formula of blue compound in the following periodic table is to be identified.
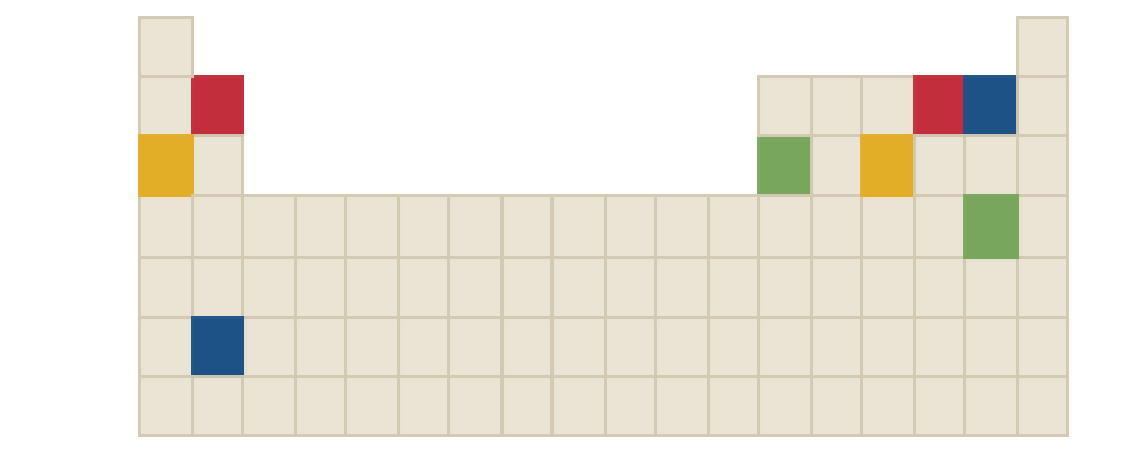
Concept Introduction:
Ions are formed by the loss or gain of electrons. The formation of ion requires the presence of two elements, these two elements are: one is metal atom and another one is non-metal atom. Metal atom loses electron and non-metal atom accepts electron.
The ratio in which positive and negative ions combine is the ratio which achieves charge neutrality for the resulting compound.
There three rules to remember while writing the chemical formulas. They are as follows:
- First write the symbol for positive ion.
- The charges of the ions are not shown in the formula.
- The numbers in the formula give the combining ratio for the ions.
(c)
Interpretation:
The chemical formula of yellow compound in the following periodic table is to be identified.
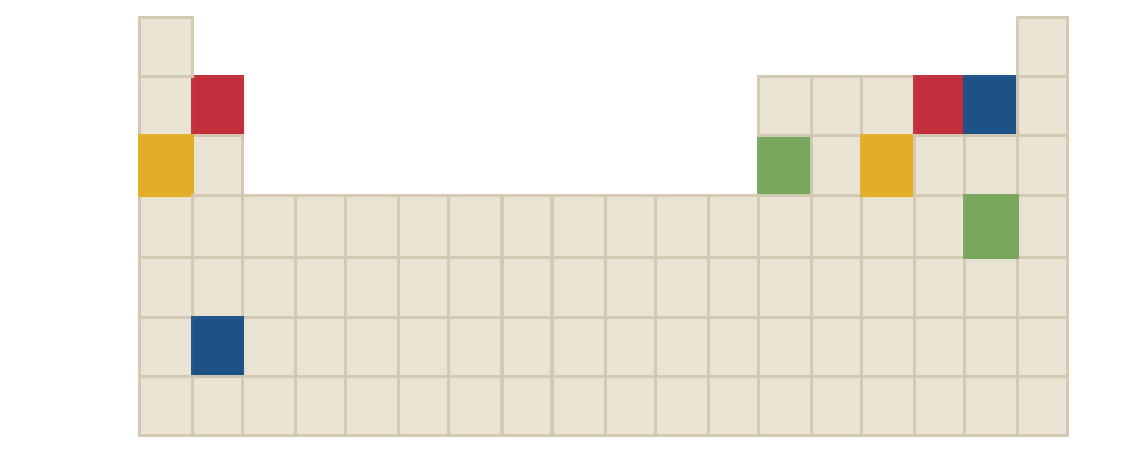
Concept Introduction:
Ions are formed by the loss or gain of electrons. The formation of ion requires the presence of two elements, these two elements are: one is metal atom and another one is non-metal atom. Metal atom loses electron and non-metal atom accepts electron.
The ratio in which positive and negative ions combine is the ratio which achieves charge neutrality for the resulting compound.
There three rules to remember while writing the chemical formulas. They are as follows:
- First write the symbol for positive ion.
- The charges of the ions are not shown in the formula.
- The numbers in the formula give the combining ratio for the ions.
(d)
Interpretation:
The chemical formula of green compound in the following periodic table is to be identified.
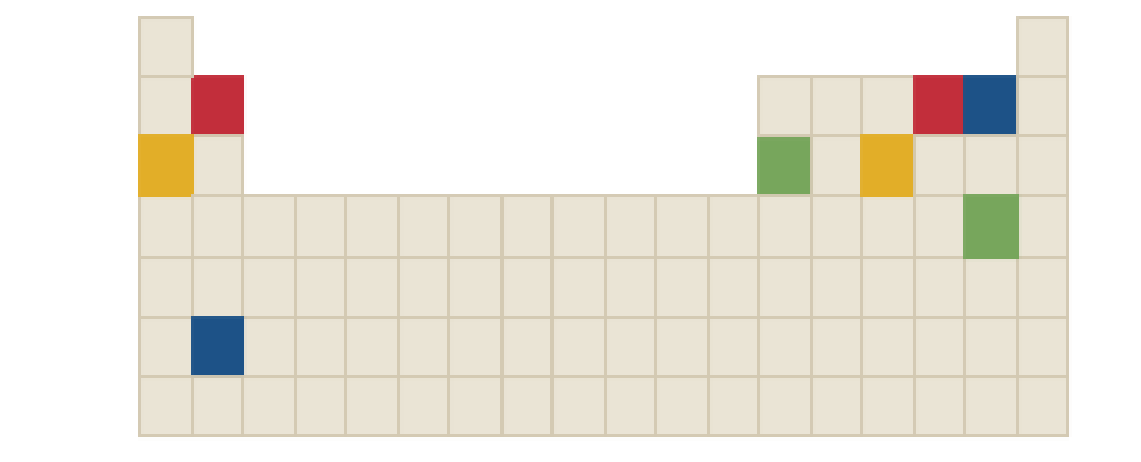
Concept Introduction:
Ions are formed by the loss or gain of electrons. The formation of ion requires the presence of two elements, these two elements are: one is metal atom and another one is non-metal atom. Metal atom loses electron and non-metal atom accepts electron.
The ratio in which positive and negative ions combine is the ratio which achieves charge neutrality for the resulting compound.
There three rules to remember while writing the chemical formulas. They are as follows:
- First write the symbol for positive ion.
- The charges of the ions are not shown in the formula.
- The numbers in the formula give the combining ratio for the ions.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
General, Organic, and Biological Chemistry Seventh Edition
- 3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward1. Which one(s) of these can be oxidized with CrO3 ? (could be more than one) a) triphenylmethanol b) 2-pentanol c) Ethyl alcohol d) CH3 2. Write in all the product(s) of this reaction. Label them as "major" or "minor". 2-methyl-2-hexanol H2SO4, heatarrow_forward3) Determine if the pairs are constitutional isomers, enantiomers, diastereomers, or mesocompounds. (4 points)arrow_forward
- In the decomposition reaction in solution B → C, only species C absorbs UV radiation, but neither B nor the solvent absorbs. If we call At the absorbance measured at any time, A0 the absorbance at the beginning of the reaction, and A∞ the absorbance at the end of the reaction, which of the expressions is valid? We assume that Beer's law is fulfilled.arrow_forward> You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: 1. ☑ CI 2. H3O+ O Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. Explanation Check ? DO 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardDon't use ai to answer I will report you answerarrow_forward
- Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence pointarrow_forwardWhat is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward
- 7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forwardIndicate the compound formula: dimethyl iodide (propyl) sulfonium.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





