
Boilers are used in most chemical plants to generate steam for various purposes, such as to preheat process streams fed to reactors and separation units. In one such process, steam and a cold process fluid are fed to a heat exchanger where enough energy is transferred from the steam to cause a large fraction of it to condense. The uncondensed steam is vented to the atmosphere, and the liquid condensate is recycled to a deaerator into which another liquid stream (makeup water) is fed. The makeup water contains some dissolved impurities and other chemicals that help prevent deposition of solids on boiler walls and heating elements, which would lead to a reduction in operating efficiency and eventually to safety hazards, possibly including explosions. The liquid leaving the deaerator is the feed to the boiler. In the boiler, most of the water in the feed evaporates to form steam, and some of the impurities in the feedwater precipitate to form solid particles suspended in the liquid (kept in suspension by the chemical additives in the makeup w ater). The liquid and suspended solids are drawn oft' as boiler blowdown, either in manual bursts or w ith a continuous blowdown system.
A diagram of the system is shown below. The symbol I is used for combined impurities and chemical additives. The makeup water contains 1.0 kg 1/2.0 X 103 kg HjO, and the ratio in the blowdown is 1.0 kg 1/3.5 X 102 kg HiO. Of the steam fed to the heat exchanger, 76% is condensed.
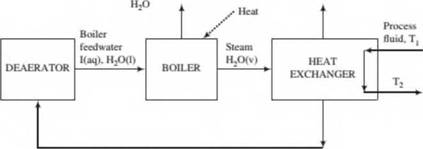
- In your own words, describe why makeup water, chemical additives to the makeup water, and blowdown are necessary in this process. Speculate on the probable disadvantage of making the 1/HsO ratio in the blowdown (I) too small, and (ii) too large.
- Assume a basis of calculation and draw and fully label a flow chart of the process. (When you draw the heat exchanger you can omit the process fluid, which plays no role in the problem.)
- Carry out a degree-of-freedom analysis and outline a solution procedure (which equations would you write in which order to calculate all of the unknow ns on the chart?).
(d, Calculate the ratio (mass of makeup water/lOOkg steam produced in boiler) and the percentage of the boiler feedwater taken off as blowdown.
(c) A proposal has been made to use highly purified water as makeup. List the benefits that would result from doing so and the most likely reason for not doing it.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Elementary Principles of Chemical Processes 4e Binder Ready Version + WileyPLUS Registration Card (Wiley Plus Products)
Additional Engineering Textbook Solutions
Starting Out with Python (4th Edition)
Concepts Of Programming Languages
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Management Information Systems: Managing The Digital Firm (16th Edition)
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
Modern Database Management
- #3 Orthonitroanaline (an important intermediate in dyes - called fast orange) is formed from the reaction of orthonitroanaline (ONCB) and aqueous ammonia. This liquid phase reaction is first order in both ONCB and ammonia with k = 0.0017 m³/kmol·min at 188 °C. The initial entering concentration of ONCB and ammonia are 1.8 kmol/m³ and 6.6 kmol/m³, respectively. ONCB is used as the basis of calculation. NO2 CI NO2 NH₂ + NHCI +2NH₂ a) Express the concentration of each species solely as a function of conversion.arrow_forward4. (15 pts)A chemical project with a fixed capital investment without land of $250,000. The operation of the chemical project starts at the end of year 1 with 8-years of project lifetime. The estimated revenue per year is $90,000, the estimated cost of manufacture without depreciation over the project lifetime is $30,000/yr, and the taxation rate is 40%. a. Please determine the yearly depreciation values using the standard MACRS method assuming surplus value of $5,000. b. Please determine the net profit for operation year 1, 5, and 8.arrow_forward2. (10 pts) You got a loan of $300,000 from a bank for your new house at a yearly interest rate of 6%, compounded monthly. How much do you pay total to the bank if the loan is 15 years? How much do you pay total to the bank if the loan is 30 years? 3. (10 pts) You got a 5-year loan of $50,000 to buy a BMW car at a yearly interest rate of 6% Please calculate your monthly payment if it is compounded monthly? Please calculate your quarterly payment if it is compounded quarterly?arrow_forward
- A buffer solution is made by mixing 0.1 M acetic acid (HA) and 0.05 M sodium acetate (A⁻). The pKa of acetic acid is 4.76. Due to an experimental error, the actual pH was not recorded, and we need to solve for the concentration of the conjugate base (A⁻) given that the desired pH should be 4.90. Use the Bisection Method to find the concentration of A.arrow_forward1. (15) John had an loan plan shown in the following discrete cash flow diagram: $4,000 $6,000 GI $2,000 5 7 1 2 3 4 $3,000 $4,000 ? Years a. Please describe this diagram in terms of borrowing and payback. b. How much does John need to pay to totally payoff the loan at the end of year 8 if the interest rate is 8%? c. If John pays the sam amount of money at year 8, how much can John borrow at year 0 without paying back in between with the same interest rate?arrow_forwardA buffer solution is made by mixing 0.1 M acetic acid (HA) and 0.05 M sodium acetate (A⁻). The pKa of acetic acid is 4.76. Due to an experimental error, the actual pH was not recorded, and we need to solve for the concentration of the conjugate base (A⁻) given that the desired pH should be 4.90. Use the Bisection Method to find the concentration of A.arrow_forward
- 1. Liquid heptane is stored in a 100,000-L storage vessel that is vented directly to air. The heptane is stored at 25°C and 1 atm pressure. The liquid is drained from the storage vessel and all that remains in the vessel is the air saturated with heptane vapor. a. Is the vapor in the storage vessel flammable? b. What is the TNT equivalent for the vapor remaining in the vessel? c. If the vapor explodes, what is the overpressure 50 m from the vessel? d. What damage can be expected at 50 m?arrow_forward2. You have decided to use a vacuum purging technique to purge oxygen from a reactor vessel to reduce the concentration to 2.0% (mol). The reactor is 18 ft diameter and 40 ft tall. The temperature is 80°F. Assume that the vacuum purge goes from atmospheric pressure to 10.0 psia. How many purge cycles are required and how many total moles of nitrogen must be used? Assume the purge is done with pure nitrogen. 3. If the purging described in problem 2 takes place using nitrogen that has 1% (mol) oxygen in it, how many vacuum purge cycles are required? How many total moles of the inert gas must be used? 4. If the purging described in problem 2 is done by way of a "sweep-through" purge instead of a vacuum purge, for how long (in minutes) must the inert gas flow through the vessel if there is a 20 psig supply of pure nitrogen available at 150 CFM (ft³/min)? How much nitrogen must be used (lbm)?arrow_forward5. Look at Figure 7-14. Determine the voltage developed between the steel nozzle and the grounded vessel, and how much energy is stored in the nozzle. Explain the potential hazards for cases A and B from the following table: Case A Case B Hose length (ft) 75 75 Hose diameter (in) 2.0 2.0 Flow rate (gpm) 30 30 Liquid conductivity (mho/cm) 2x10-8 1x10-14 Dielectric constant 2.3 25 Density (g/cm³) 0.8 0.9 6. In Problem 5, case B, what would be the most effective way to reduce the potential hazards in this situation?arrow_forward
- 2. You have decided to use a vacuum purging technique to purge oxygen from a reactor vessel to reduce the concentration to 2.0% (mol). The reactor is 18 ft diameter and 40 ft tall. The temperature is 80°F. Assume that the vacuum purge goes from atmospheric pressure to 10.0 psia. How many purge cycles are required and how many total moles of nitrogen must be used? Assume the purge is done with pure nitrogen.arrow_forwardAn 8-foot ion exchange bed needs to be backwashed with water to remove impurities. The particles have a density of 1.24 g/cm³ and an average size of 1.1 mm. Calculate: a. The minimum fluidization velocity using water at 30°C? b. The velocity required to expand the bed by 30%? Assumptions: The ion exchange bed particles are spherical (sphericity = 1.1), and the minimum fluidization porosity (ɛM) is 0.3. Notes: At 30°C, the viscosity (μ) of water is 0.797 cP, and the density (ρ) is 0.995 g/cm³.arrow_forwardfluidized bed reactor uses a solid catalyst with a particle diameter of 0.25 mm, a bulk density of 1.50 g/mL, and a sphericity of 0.90. Under packed bed conditions, the porosity is 0.35, and the bed height is 2 m. The gas enters from the bottom of the reactor at a temperature of 600°C, with a viscosity of 0.025 cP and a density of 0.22 lb/ft³. At minimum fluidization, the porosity reaches 0.45. Calculate: a. The minimum superficial velocity (VM) of the gas entering the fluidized column. b. The bed height if V = 2 VM c. The pressure drop under conditions where V =2.5 VMarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





