
INTRO.TO CHEM.ENGR.THERMO.-EBOOK>I<
8th Edition
ISBN: 9781260940961
Author: SMITH
Publisher: INTER MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4, Problem 4.32P
Interpretation Introduction
Interpretation:
The standard heat of combustion of fuel oil at 25 oC = 298 K should be calculated.
Concept Introduction:
From the definition of heat flow, heat is related to internal energy and enthalpy of reaction as follows:
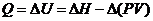
Here, Q is heat released,
Also, the heat of combustion at 298K can be calculated using the following equation:
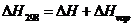
Here,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What kind of boundary must a system have to undergo the stated Interaction with its surroundings if possible ( mention the 3 qualities of the boundary in each case
A. WORK INTERACTIONS ONLY
B. MASS AND HEAT INTERACTIONS ONLY
C. HEAT INTERACTIONS ONLY IS THIS POSSIBLE, EXPLAIN.
D. WORK AND MASS INTERACTIONS ONLY.
E. WORK AND HEAT INTERACTIONS ONLY
F. MASS INTERACTIONS ONLY. IS THIS POSSIBLE OR NOT. EXPLAIN
Answer the questions
Figure below shows a portion of a fire protection system in which apump draws water at 60 F from a reservoir and delivers it to point B at the flow rate of 1500 gal/min
a). Calculate the required height of the water level in the tank in order to maintain 5.0 psig pressure at point A. Answer: h = 12,6 ft
b). Assuming that the pressure at A is 5.0 psig, calculate the power delivered by the pump to the water in order to maintain the pressure at point B at 85 kPa. Include energy lost due to friction but neglect any other energy losses. P₁ =19,2 hp
Chapter 4 Solutions
INTRO.TO CHEM.ENGR.THERMO.-EBOOK>I<
Ch. 4 - Prob. 4.1PCh. 4 - Prob. 4.2PCh. 4 - Prob. 4.3PCh. 4 - Prob. 4.4PCh. 4 - Prob. 4.5PCh. 4 - Prob. 4.6PCh. 4 - Prob. 4.7PCh. 4 - Prob. 4.8PCh. 4 - Prob. 4.9PCh. 4 - Prob. 4.10P
Ch. 4 - Prob. 4.11PCh. 4 - Prob. 4.12PCh. 4 - Prob. 4.13PCh. 4 - Prob. 4.14PCh. 4 - Prob. 4.15PCh. 4 - Prob. 4.16PCh. 4 - Prob. 4.17PCh. 4 - Prob. 4.18PCh. 4 - Prob. 4.19PCh. 4 - Prob. 4.20PCh. 4 - Prob. 4.21PCh. 4 - Prob. 4.22PCh. 4 - Prob. 4.23PCh. 4 - Prob. 4.24PCh. 4 - Develop a general equation for the standard heat...Ch. 4 - Compute the standard heat of reaction for each of...Ch. 4 - Prob. 4.27PCh. 4 - Prob. 4.28PCh. 4 - Prob. 4.29PCh. 4 - Prob. 4.30PCh. 4 - Prob. 4.31PCh. 4 - Prob. 4.32PCh. 4 - Prob. 4.33PCh. 4 - Prob. 4.34PCh. 4 - Ethylene gas and steam at 320°C and atmospheric...Ch. 4 - Prob. 4.36PCh. 4 - A fuel consisting of 75 mol-% methane and 25 mol-%...Ch. 4 - Prob. 4.38PCh. 4 - Prob. 4.39PCh. 4 - Prob. 4.40PCh. 4 - An equimolar mixture of nitrogen and acetylene...Ch. 4 - Prob. 4.42PCh. 4 - A gas consisting only of CO and N2 is made by...Ch. 4 - Prob. 4.44PCh. 4 - Prob. 4.45PCh. 4 - Prob. 4.46PCh. 4 - Prob. 4.47PCh. 4 - Prob. 4.48PCh. 4 - Prob. 4.49PCh. 4 - Prob. 4.50PCh. 4 - Quantitative thermal analysis has been suggested...Ch. 4 - Prob. 4.52PCh. 4 - Prob. 4.53PCh. 4 - The oxidation of glucose provides the principal...Ch. 4 - Prob. 4.55P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Water at 60° F is being pumped from a stream to a reservoir whose surface is 210 ft above the pump. The pipe from the pump to the reservoir is an 8-in Schedule 40 steel pipe 2500 ft long. The pressure at the pump inlet is - 2,36 psig. If 4.00 ft³/s is being pumped, a). Compute the pressure at the outlet of the pump. Answer: 0,997 MPa b). Compute the power delivered by the pump to the water. Answer: 151 hp Consider the friction loss in the discharged line, but neglect other lossesarrow_forward1. Consider a mixture of 2.5.0% ethane, 2.0% butane, and 1.7% n-pentane by volume.a. Estimate the LFL and UFL of the mixture. Is it flammable?b. Estimate the LOC for this mixture.arrow_forwardEstimate the LFL and UFL for propylene using Equations 6-10 and 6-11 in the textbook,and compare these to the experimental values given in the table in Appendix B.arrow_forward
- 1. Determine the minimum compression ratio required to raise the temperature of air overhexane to its AIT. Assume an initial temperature of 20°C.2. Ethanol is kept in a storage vessel that is vented with air (at 25°C and 1 atm). Is theequilibrium mixture of vapor above the liquid and air flammable? What if the liquid isacetone instead?arrow_forwardHydrogenation of Ethylbenzene to Styrene Reaction: C₈H₁₀ → C₈H₈ + H₂ΔHᵣ°(300°C) = -124 kJ/mol (exact value unknown) Process Description: The basis is 1000 kg/h of separated styrene. The reaction conversion rate is 35%. The temperature increase in heat exchanger 2 is adiabatic. A fresh stream of pure ethylbenzene (25°C) enters a mixing vessel, where it is combined with a recycle stream (from the distillation column, as explained later), which also consists of pure ethylbenzene at 25°C. After mixing, the stream is sent to a heat exchanger (HX1), where the mixture is heated to 200°C. Next, the mixture enters an adiabatic heat exchanger (HX2), where it is further heated to 300°C by adding steam (at 350°C). This steam is used to prevent side reactions and carbon deposition in the reactor. The heated mixture is then fed into the reactor, where the reaction takes place with a conversion rate of 35%. As a result, the mixture cools down to 260°C. The resulting mixture is then sent to HX4, where…arrow_forwardChemical Engineering Questionarrow_forward
- 4.16 aarrow_forward8. The thermal decomposition of nitric oxide at elevated temperatures 2NO → N₂+02 has been studied in a batch reactor where at temperatures below 2000K the rate expression that applies to low conversions is: r = kCm05 Co At high conversions, or when the initial mixture contains a high concentration of O2 the rate expression is given by: r = k' Cм0.5 C15C0,5 To explain these kinetics the following chain reaction mechanism has been proposed: Initiation: Propagation: 2NON₂O +0 k₂ E1=272.0 kJ/mol 0+ NO O₂+ N E₂-161.0 kJ/mol N+NO N₂+0 E3-1.4 kJ/mol K4 20+ MO₂+M E4=14.0 kJ/mol ks Termination: where M is any molecule capable of the energy transfer necessary to stabilize the oxygen molecule. Once appreciable amounts of O2 are present in the reaction mixture, the initiation reaction that is the primary source of atomic oxygen is no longer the first reaction. Instead, the following reaction begins to dominate the chain initiation process: Initiation (high O2): ks NO +0₂ NO₂+0 E5=198.0 kJ/mol a.…arrow_forward2:41 2) If the number-average degree of polymerization for styrene obtained by the bulk polymerization at 25°C is 5,000, what would be the number-average degree of polymerization if conducted in a 10% solution in toluene (900g of toluene per 100 g of styrene) under otherwise identical conditions? State any assumptions that are needed. (see Table 2-4). Table 2-4 Representative Values of Chain-Transfer Constants Monomer Styrene Chain-Transfer Agent T (°C) C x 104 Styrene 25 bas 0.279 * 50 0.35-0.78 Polystyrene 50 1.9-16.6 Benzoyl peroxide 50 0.13 Toluene 60 0.125 Methyl methacrylate Methyl methacrylate 30 0.117 70 0.2 Poly(methyl methacrylate) 50 0.22-1000 Benzoyl peroxide 50 0.01 Toluene 40 0.170 3) 2 3) Methyl methacrylate is copolymerized with 2-methylbenzyl methacrylate (M₁) in 1,4- dioxane at 60°C using AIBN as the free-radical initiator. (a) Draw the repeating unit of poly(2-methylbenzyl methacrylate). (b) From the data given in the table below, estimate the reactivity ratios of…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The