
Concept explainers
Use a search engine such as Google to find information about Gordon E. Moore and Moore’s law, the famous law about technological advances that he proposed.
(a) What is Moore’s law? Give a brief description in your own words.
(b) Who is Gordon E. Moore? What was his position at the time he first proposed Moore’s law? What company did he later cofound? With whom did he cofound this company?
(c) In what field did Gordon E. Moore obtain his BS degree? At what university did he receive his BS degree? Where did he obtain his PhD degree? In what field was his PhD degree?
(d) What Nobel Prize-winning physicist gave Gordon E. Moore his first job opportunity?
(e) What was the number of the first microprocessor developed at Moore’s company and how many transistors did it have! When was it introduced!
(f) Read the article by Thomas Friedman on Moore’s law, and watch the video of his interview of Gordon E. Moore.9 In what year in what publication did Moore make his prediction? What was the most important lesson that Moore learned from his law? What stimulated his interest in science and engineering? What does Moore see as the biggest problem in science? How would you describe thestatus of Moore’s Law today!
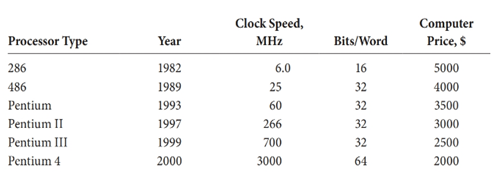
(g) One important benchmark ofcomputational progress is the performance-to-price ratio (PPR) of computers.10 The PPR is the number of bits per word divided by the product of cycle time (1/clockspeed) and price. The original IBM PC (1981) with an 8bit word length, a 4.77 MHz clock, and a pricetag of $5000 came in with a PPR of ~7600. Computers based on other processors available in 2000 are listed in the following table.10 Calculate the PPR of each of these computers. Does Moore’s law hold forthe PPR? How did you come to your conclusion?
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Principles of Instrumental Analysis, 6th Edition
- 6. Choose the compound that will produce the spectrum below and assign the signals as carbonyl, aryl, or alkyl. 100 ō (ppm) 50 0 7. 200 150 Assign all of the protons on the spectrum below. 8. A B 4 E C 3 ō (ppm) 2 1 0 Choose the compound that will produce the spectrum below and assign the signals to the corresponding protons. OH 6 OH 3 2 1 0 4 ō (ppm)arrow_forwardIn the Thermo Fisher application note about wine analysis (Lesson 3), the following chromatogram was collected of nine components of wine. If peak 3 has a retention time of 3.15 minutes and a peak width of 0.070 minutes, and peak 4 has a retention time of 3.24 minutes and a peak width of 0.075 minutes, what is the resolution factor between the two peaks? [Hint: it will help to review Lesson 2 for this question.] MAU 300 200 T 34 5 100- 1 2 CO 6 7 8 9 0 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 4.0 4.2 4.4 4.6 4.8 5.0 5.2 Minutes 3.22 0.62 1.04 O 1.24arrow_forwardThe diagram shows two metals, A and B, which melt at 1000°C and 1400°C. State the weight percentage of the primary constituent (grains of C) that would be obtained by solidifying a 20% alloy of B. 1000°C a+L L+C 900°С 12 α a+C 45 1200 C L+y 140096 C+Y a+ß 800°C 700°C C+B 96 92 a+B 0 10 20 30 40 50 60 70 80 90 100 A % peso B Barrow_forward
- 8. Choose the compound that will produce the spectrum below and assign the signals to the corresponding protons. 2 4 3 ō (ppm) OH 4 6 6 СОН 2 1 0arrow_forward7. Assign all of the protons on the spectrum below. A B 2 C E 2 1 3 6 4 3 2 1 0arrow_forwarde. If (3R,4R)-3,4-dichloro-2,5-dimethylhexane and (3R,4S)-3,4-dichloro-2,5-dimethylhexane are in a solution at the same concentration, would this solution be expected to rotate plane polarized light (that is, be optically active)? Please provide your reasoning for your answer. [If you read this problem carefully, you will not need to draw out the structures to arrive at your answer...]arrow_forward
- 1. How many neighbors does the proton that produces the multiplet below have? 2. 3. اللـ Draw a partial structure from the multiplet below. (The integration of the multiplet is 6) M Using the additivity constants found in appendix G of your lab manual, calculate the approximate chemical shifts of the protons indicated below. (Show your work!!!) B A Br SHarrow_forward1) Suppose 0.1 kg ice at 0°C (273K) is in 0.5kg water at 20°C (293K). What is the change in entropy of the ice as it melts at 0°? To produce the original "water gas" mixture, carbon (in a combustible form known as coke) is reacted with steam: 131.4 kJ + H20(g) + C(s) → CO(g) + H2(g) From this information and the equations in the previous problem, calculate the enthalpy for the combustion or carbon to form carbon dioxide. kindly show me how to solve this long problem. Thanksarrow_forward4. An 'H-NMR of a compound is acquired. The integration for signal A is 5692 and the integration for signal B is 25614. What is the simplest whole number ratio of protons for signals A and B? (Show your work!!!) 5. Assign the carbons in the NMR below as either carbonyl, aromatic, or alkyl. 200 150 100 50 ō (ppm) 1arrow_forward
- Speaking of composite materials, indicate the correct option:(A). Composite materials can only be: metal-polymer or polymer-polymer.(B). Composite materials can be made up of particles, but not fibers or sheets.(C). When the reinforcing particles are uniformly distributed in a composite material, there may be a greater tendency for it to have isotropic properties.(D). None of the above is correct.arrow_forwardIf we are talking about viscoelastic modulus or viscoelastic relaxation modulus in polymers, indicate the correct option.(A). It reports the variation of elastic behavior as a function of time.(B). It is only useful for defining its glass transition temperature.(C). It only allows us to define the polymer degradation temperature.(D). Neither option is correct.arrow_forwardWhen natural light falls perpendicularly on a material A, it has a reflectivity of 0.813%. Indicate the value of the refractive index.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





