
Fuel oils contain primarily organic compounds and sulfur. The molar composition of the organic fraction of a fuel oil may be represented by the formula
the mass fraction of sulfur in the fuel is
As (kg S/kg fuel); and the percentage excess air, Pn, is defined in terms of the theoretical air required to burn only the carbon and hydrogen in the fuel.
- For a certain high-sulfur No. 6 fuel oil, p = 0.71, 2, SO2, and H2O and expressing the SO2 fraction as ppm (mol SO2/I()6mol dry gas).
- Create a spreadsheet to calculate the mole fractions of the stack gas components on a dry basis for specified values of p, q, r, as, and PXi. The output should appear as follows:
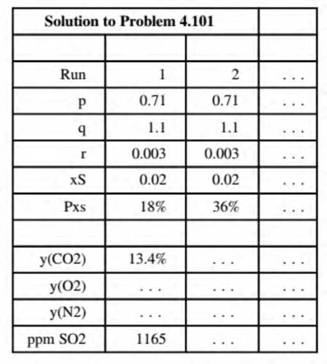
(Rows below the last one shown can be used to calculate intermediate quantities.) Execute enough runs (including the two show n above) to determine the effect on the stack gas composition of each of the five input parameters. Then for the values of p, q, r. and As given in Part (a), find the minimum percentage excess air needed to keep the dry-basis SO2 composition below 700 ppm. (Make this the last run in the output table.)
You should find that for a given fuel oil composition, increasing the percentage excess air decreases the SO2 concentration in the stack gas. Explain w hy this should be the case.
- Someone has proposed using the relationship betw een PX5 and ppm SO2 as the basis of a pollution control strategy. The idea is to determine the minimum acceptable concentration of SO2 in the stack gas, then run with the percentage excess air high enough to achieve this value. Give several reasons w hy this is a poor idea.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ELEM.PRIN.OF CHEM.PROCESS-ACCESS
Additional Engineering Textbook Solutions
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Mechanics of Materials (10th Edition)
Introduction To Programming Using Visual Basic (11th Edition)
Concepts Of Programming Languages
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
- A semi-truck tire is inflated to 110 psig with nitrogen. What will be the initial gas discharge ratein lbm/s due to a 1/16-inch diameter hole? Assume at temperature of 80℉ and an ambientpressure of 1 atm.arrow_forward# 4 The reaction, AB, is to be carried out isothermally in a continuous flow reactor. The entering volumetric flow rate, vo is 10 L/h and is constant (v=vo). Calculate both the CSTR and PFR volumes necessary to reduce the entering concentration of species A from CAD to CA = 0.01 CAO when the entering molar flow rate of species A is 5 mol/h. (a) This reaction is a second order reaction. The reaction rate constant, k is given as 300 L/mol.h. (b) This reaction is a zeroth order reaction. The reaction rate constant, k is given as 0.05 mol/h.L.arrow_forward#3 Using the initial rates method and the given experimental data below to determine the rate law and the value of the rate constant for the reaction, as shown below. All trials are performed at the same temperature. 2NO + Cl2 → 2NCOCI Trial [NO] (mol/L) [Cl₂] (mol/L) Initial rates (mol/L.s) 1 0.10 0.10 0.00300 2 0.10 0.15 0.00450 3 0.15 0.10 0.00675arrow_forward
- #2 The reaction rate constant at temperature, T₁, is 15 mol/L-s while at the reaction rate constant changed to 7 mol/L-s when temperature changed to T2 at 398 K. What is T₁? Given the activation energy is 600 kJ/mol. Assume at this temperature interval, pre-exponential factor and activation energy are constant.arrow_forward#1 Chloral is consumed at a rate of 10 mol/L-s when reacting with chlorobenzene to form DDT and water in the reaction given below. Determine: i) the rate of disappearance of chlorobenzene. ii) the rate of formation of DDT. CCI CHO (Chloral) + 2C6H5Cl (Chlorobenzene) → (C6H4Cl)2CHCCI 3 (DDT) + H2Oarrow_forward#5 The irreversible liquid phase second order reaction, 2A → B, is carried out in a CSTR. The entering concentration of A, CAD is 2 mol/L, and the exit concentration of A, CA is 0.1 mol/L. The volumetric flow rate, vo, is at 3 L/s and is constant (v=vo). The reaction rate constant, k is 0.03 L/mol's. What is the corresponding reactor volume?arrow_forward
- Problem 9.11 An 80 mm long line MN has its end M 15 mm in front of the V.P. The distance between the ends projector is 50 mm. The front view is parallel to and 20 mm above reference line. Draw the projections of the line and determine its inclination with the V.P. Also, locate the traces. Interpretation Front view of a line is parallel to xy, therefore, 1. The line is parallel to the H.P. 2. The top view of the line has true length. 3. The front view has projected length equal to the distance be- tween the projectors. Construction Refer to Fig. 9.11. 1. Draw a reference line xy. Mark point m' 20 mm above xy and point m 15 mm below xy. 2. Draw a 50 mm long line m'n' parallel to xy. 3. Draw an arc with centre m and radius 80 mm to meet projec- tor from point n' at point n. Join mn to represent the top view. Determine its inclination with xy as the inclination of line MN with the V.P. Here = 51°. 4. Traces Extend line mn to meet xy at point v. Project point v to meet m'n' produced at…arrow_forwardoh 30 20 D и D P 60 60 80arrow_forward⑤ b Δε m ab C 40arrow_forward
- Problem 10.16 An isosceles triangle of base 40 mm and altitude 54 mm has its base in the V.P. The surface of the plane is inclined at 50° to the V.P. and perpendicular to the H.P. Draw its projections. Construction Refer to Fig. 10.17. An isosceles triangle has its base in the V.P., so con- sider that initially the triangle ABC is placed in the V.P. with base AB perpendicular to the H.P. 1. First stage Draw a triangle a'b'c' keeping a'b' perpendicular to xy to represent the front view. Project the corners to xy and obtain ac as the top view. 2. Second stage Reproduce the top view of first stage keeping ab on xy and ac inclined at 50° to xy. Obtain new points a', b' and c' in the front view by joining the points of intersection of the vertical projectors from a, b and c of the second stage with the corresponding horizontal locus lines from a', b' and c' of the first stage. Join a'b'c' to represent the final front view. Here, the front view is an equilateral triangle of side 40 mm. X 54…arrow_forward%9..+ ۱:۱۹ X خطأ عذرا ، الرقم الذي أدخلته خاطئ. يرجى إدخال رقم بطاقة الشحن الصالحة والمحاولة مرة أخرى. رصيد هاتفك قم بمسح الرمز = رقم بطاقة التعبئة 7794839909080 رمز مكون من 13 او 14 رقماً طريقة إعادة التعبئة قم باعادة تعبئة الرصيد إعادة تعبئة الإنترنت إعادة تعبئة الرصيد O >arrow_forwardProblem 10.14 A hexagonal plane of side 30 mm has a corner in the V.P. The surface of the plane is inclined at 45° to the V.P. and perpendicular to the H.P. Draw its projections. Assume that the diagonal through the corner in the V.P. is parallel to the H.P. d' a 2 b b.f C' c.e b 'C' H.P. (a) V.P E HEX 30 e' O' d' a a' b' C' b' X y a b,f c,e d b,f (b) c,earrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





