
A liquid-phase chemical reaction A -* B takes place in a well-stirred tank. The concentration of A in the feed is f-AO (mol/m3), and that in the tank and outlet stream is CA (mol/nr). Neither concentration varies with time. The volume of the tank contents is V(m3) and the volumetric flow rale of the inlet and outlet streams is V (m7s). The reaction rate (the rate at which A is consumed by reaction in the lank) is given by the expression
r(mol A consumed/s) = JtVCA
where k is a constant.
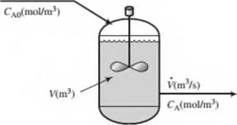
- Is this process continuous, batch, or semibatch? Is it transient or steady-state?
- What would you expect the reactant concentration CA to equal if k = 0 (no reaction)? What should it approach if k —» oo (inflnitely rapid reaction)?
- Write a differential balance on A, stating which terms in the general balance equation (accumulation = input + generation — output — consumption) you discarded and why you discarded them. Use the balance to derive the following relation between the inlet and outlet reactant concentrations:

Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ELEM.PRIN.OF CHEM.PROCESS-ACCESS
Additional Engineering Textbook Solutions
Electric Circuits. (11th Edition)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Starting Out with Python (4th Edition)
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
- Show reaction mechanism. don't give Ai generated solutionarrow_forward7. Draw the Lewis structures and molecular orbital diagrams for CO and NO. What are their bond orders? Are the molecular orbital diagrams similar to their Lewis structures? Explain. CO Lewis Structure NO Lewis Structure CO Bond Order NO Bond Order NO Molecular Orbital Diagram CO Molecular Orbital Diagramarrow_forward5. The existence of compounds of the noble gases was once a great surprise and stimulated a great deal of theoretical work. Label the molecular orbital diagram for XeF (include atom chemical symbol, atomic orbitals, and molecular orbitals) and deduce its ground state electron configuration. Is XeF likely to have a shorter bond length than XeF+? Bond Order XeF XeF+arrow_forward
- 6. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+ B22+, B2, C22, B22 and N22+ Molecular Orbital Diagram B2 C22- B22- N22+ Which molecule is paramagnetic?arrow_forward3. Put the following species in order of increasing bond length by using molecular orbital diagrams and calculating their bond orders: F2, F2, F2+ Molecular Orbital Diagram F2 F2 F2+ Bond Order Shortest bond: Longest bondarrow_forward3. Put the following species in order of increasing bond length by using molecular orbital diagrams and calculating their bond orders: F2, F2, F2+ Molecular Orbital Diagram F2 F2 F2+ Bond Orderarrow_forward
- 4. The superoxide ion, Oz, plays an important role in the ageing processes that take place in organisms. Judge whether Oz is likely to have larger or smaller dissociation energy than 02. Molecular Orbital Diagram 02 02 Does O2 have larger or smaller dissociation energy?: Bond Orderarrow_forward1. How many molecular orbitals can be built from the valence shell orbitals in O2?arrow_forwardSho reaction mechanism. Don't give Ai generated solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





