
Chemistry Atoms First2e
2nd Edition
ISBN: 9781947172647
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 37E
Iii (he Lewis structures listed here, M and X represent various elements iii the third period of the periodic table. Write the formula of each compound using the
(a) 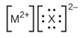
(b) 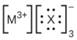
(c) 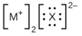
(d) 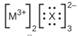
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
What is the IUPAC name of the following compound?
CH₂CH₂
H
CI
H₂CH₂C
H
CH₂
Selected Answer:
O
(35,4R)-4 chloro-3-ethylpentane
Correct
Chapter 4 Solutions
Chemistry Atoms First2e
Ch. 4 - Does a cation gain protons to form a positive...Ch. 4 - Iron(III) sulfate [Fe2( SO 4)3] is composed of...Ch. 4 - Which of the following atoms would be expected to...Ch. 4 - Which of the following atoms would be expected to...Ch. 4 - Predict the charge on the monatomic ions formed...Ch. 4 - Predict the charge on the monatomic ions formed...Ch. 4 - Write the electron configuration for each of the...Ch. 4 - Write the electron configuration for the monatomic...Ch. 4 - Write out the full electron configuration for each...Ch. 4 - From the labels of several commercial products,...
Ch. 4 - Why is it incorrect to speak of a molecule of...Ch. 4 - What information can you use to predict whether a...Ch. 4 - Predict which of the following compounds are ionic...Ch. 4 - Explain the difference between a nonpolar covalent...Ch. 4 - From its position in the periodic table, determine...Ch. 4 - From its position in the periodic table, determine...Ch. 4 - From their positions in the periodic able, arrange...Ch. 4 - From their positions in the periodic table,...Ch. 4 - Which atoms can bond to sulfur so as to produce a...Ch. 4 - Which is the most polar bond? (a) CC (b) CH (c) NH...Ch. 4 - Identify the more polar bond in each of the...Ch. 4 - Which of the following molecules or ions contain...Ch. 4 - Name the following compounds: (a) CsCl (b) BaO (c)...Ch. 4 - Name of the following compounds: (a) NaF (b) Rb2O...Ch. 4 - Write the formulas of the following compounds: (a)...Ch. 4 - Write the formulas of the following compounds: (a)...Ch. 4 - Write the formulas of the following compounds: (a)...Ch. 4 - Write the formulas of the following compounds: (a)...Ch. 4 - Each of the following compounds contains a metal...Ch. 4 - Each of the following compounds contains a metal...Ch. 4 - The following ionic compounds are found in common...Ch. 4 - The following ionic compounds are found in common...Ch. 4 - What are the IUPAC names of the following...Ch. 4 - Write the Lewis symbols for each of the following...Ch. 4 - Many monatomic ions are found in seawater,...Ch. 4 - Write the Lewis symbols of the ions in each of the...Ch. 4 - Iii (he Lewis structures listed here, M and X...Ch. 4 - Write the Lewis structure for the diatomic...Ch. 4 - Write Lewis structures for the following: (a) H2...Ch. 4 - Write Lewis structures for the following: (a) O2...Ch. 4 - Write Lewis structures for the following: (a) ClF3...Ch. 4 - Write Lewis structures for the following: (a) SeF6...Ch. 4 - Write Lewis structures for: (a) PO43 (b) ICl4 (c)...Ch. 4 - Correct the following statement: The bonds in...Ch. 4 - Write Lewis structures for the following molecules...Ch. 4 - Methanol, H3COH, is used as the fuel in some race...Ch. 4 - Many planets in our solar system contain organic...Ch. 4 - Carbon tetrachloride was formerly used in fire...Ch. 4 - Identify the atoms that correspond to each of the...Ch. 4 - The arrangement of atoms in several biologically...Ch. 4 - A compound with a molar mass of about 28 g/mol...Ch. 4 - A compound with a molar mass of about 42 g/mol...Ch. 4 - Two arrangements of atoms are possible for a...Ch. 4 - How are single, double, and triple bonds similar?...Ch. 4 - Write resonance forms that describe the...Ch. 4 - Write resonance forms that describe the...Ch. 4 - Write the resonance forms of ozone, Q3, the...Ch. 4 - Sodium nitrite, which has been used to preserve...Ch. 4 - In terms of the bonds present, explain why acetic...Ch. 4 - Write the Lewis structures for the following, and...Ch. 4 - Toothpastes containing sodium hydrogen carbonate...Ch. 4 - Determine the formal charge of each element in the...Ch. 4 - Determine the formal charge of each element in the...Ch. 4 - Calculate the formal charge of chlorine in the...Ch. 4 - 54. Calculate the formal charge of each element in...Ch. 4 - Draw all possible resonance structures for each of...Ch. 4 - Based on formal charge considerations, which of...Ch. 4 - Based on formal charge considerations, which of...Ch. 4 - Based on formal charge considerations, which of...Ch. 4 - Draw the structure of hydroxylamine, H3NO, and...Ch. 4 - Iodine forms a series of fluorides (listed here)....Ch. 4 - Write the Lewis structure and chemical formula of...Ch. 4 - Which of the following structures would we expect...Ch. 4 - Sulfuric acid is the industrial chemical produced...Ch. 4 - Explain why the HOH molecule is bent, whereas the...Ch. 4 - What feature of a Lewis structure can be used to...Ch. 4 - Explain the difference between electron-pair...Ch. 4 - Why is the HNH angle in NH3 smaller than the HCH...Ch. 4 - Explain how a molecule that contains polar bonds...Ch. 4 - As a general rule, MX molecules (where M...Ch. 4 - Predict the electron pair geometry and the...Ch. 4 - Identify the electron pair geometry and the...Ch. 4 - What are the electron-pair geometry and the...Ch. 4 - Predict the electron pair geometry and the...Ch. 4 - Identify the electron pair geometry and the...Ch. 4 - Predict the electron pair geometry and the...Ch. 4 - Which of the following molecules and ions contain...Ch. 4 - Which of these molecules and ions contain polar...Ch. 4 - Which of the following molecules have dipole...Ch. 4 - Identify the molecules with a dipole moment: (a)...Ch. 4 - The molecule XF3 has a dipole moment. Is X boron...Ch. 4 - The molecule XCl2 has a dipole moment. Is X...Ch. 4 - Is the Cl2BBCl2 molecule polar or nonpolar?Ch. 4 - There are three possible structures for PCl2F3...Ch. 4 - Describe the molecular structure around the...Ch. 4 - Draw the Lewis structures and predict the shape of...Ch. 4 - A molecule with the formula AB2, in which A and B...Ch. 4 - A molecule with the formula AB3, in which A and B...Ch. 4 - Draw the Lewis electron dot structures for these...Ch. 4 - What is the molecular structure of the stable form...Ch. 4 - A compound with a molar mass of about 42 g/mol...Ch. 4 - Use the simulation...Ch. 4 - Use the simulation...Ch. 4 - Use the Molecule Shape simulator...Ch. 4 - Use the Molecule Shape simulator...Ch. 4 - Use the Molecule Shape simulator...
Additional Science Textbook Solutions
Find more solutions based on key concepts
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
MAKE CONNECTIONS Which chemical group is most likely to be responsible for an organic molecule behaving as a ba...
Campbell Biology (11th Edition)
1. ___ Mitosis 2. ___ Meiosis 3. __ Homologous chromosomes 4. __ Crossing over 5. __ Cytokinesis A. Cytoplasmic...
Microbiology with Diseases by Body System (5th Edition)
Complete and balance each acid-base reaction. a. HC2H3O2(aq)+Ca(OH)2(aq) b. HBr(aq)+LiOH(aq) c. H2SO4(aq)+Ba(OH...
Introductory Chemistry (6th Edition)
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
Match the following examples of mutagens. Column A Column B ___a. A mutagen that is incorporated into DNA in pl...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forwardLook at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forward
- Given 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- Concentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forwardExplain why the following names of the structures are incorrect. CH2CH3 CH3-C=CH-CH2-CH3 a. 2-ethyl-2-pentene CH3 | CH3-CH-CH2-CH=CH2 b. 2-methyl-4-pentenearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY