
Interpretation:The name and formula of compounds formed by given cation and anion needs to be determined.
Concept Introduction: An ionic compound is composed of cation and anion. Here cation is positively charged and anion carries negative charge.
The cation and anion can be composed of more than one atoms. Such types of ions are called polyatomic ions.
Explanation of Solution
Given information:
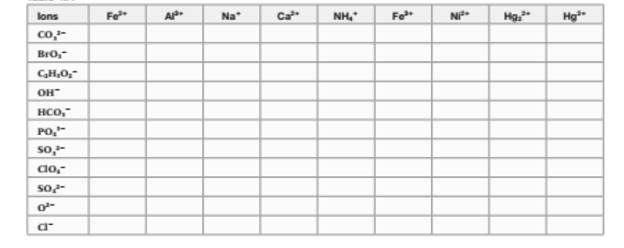
The chemical formula of any compound can be written with criss-cross method in which the valency of both cation and anion are cross multiply to get the simplest whole number of elements in the formula. Thus, the name and formula of compound are listed below;
Ferrous carbonate | Aluminum carbonate | Sodium carbonate | Calcium carbonate | Mercury (I) carbonate | |
iron(II) bromate | Aluminum (III) bromate | Sodium bromate | Calcium bromate | Mercury (II) bromate | |
Iron (II) acetate | Aluminum acetate | Sodium acetate | Calcium acetate | mercury (II) acetate | |
iron (III) hydroxide | Aluminum hydroxide | Sodium hydroxide | Calcium hydroxide | Mercury (II) hydroxide | |
Iron (II) hydrogen carbonate | Aluminum hydrogen carbonate | sodium hydrogen carbonate | calcium hydrogen carbonate | Mercury (II) hydrogen carbonate | |
Iron(II) phosphate | aluminum phosphate | sodium phosphate | calcium phosphate | mercury (II) phosphate | |
iron(II) sulfite | aluminum sulfite | sodium sulfite | calcium sulfite | mercury (II) sulfite | |
iron(II) perchlorate | aluminum perchlorate | sodium perchlorate | calcium perchlorate | Mercury (II) perchlorate | |
iron(II) sulfate | aluminum sulfate | sodium sulfate | calcium sulfate | mercury (II) sulfate | |
iron (II) oxide | aluminum oxide | sodium oxide | calcium oxide | mercury (II) oxide | |
iron (II) chloride | aluminum chloride | sodium chloride | calcium chloride | mercury (II) chloride |
Ammonium carbonate | Ferric carbonate | Nickel carbonate | Mercury (I) carbonate | |
Ammonium bromate | Iron (III) bromate | Nickel (II) bromate | Mercury (I) bromate | |
Ammonium acetate | Iron (III) acetate | Nickel (II) acetate | mercury (I) acetate | |
Ammonium hydroxide | Iron (III) hydroxide | Nickel (II) hydroxide | mercury (I) hydroxide | |
ammonium hydrogen carbonate | iron(III) hydrogen carbonate | nickel (II) hydrogen carbonate | mercury (I) hydrogen carbonate | |
ammonium phosphate | iron(II) phosphate | nickel (II) phosphate | mercury (I) phosphate | |
ammonium sulfite | iron (III) sulfite | Nickel (II) sulfite | Mercury (I) sulfite | |
ammonium perchlorate | iron(III) perchlorate | Nickel(II) perchlorate | Mercury (I) perchlorate | |
ammonium sulfate | iron(III) sulfate | Nickel (II) sulfate | Mercury (I) sulfate | |
Ammonium oxide | iron (III) oxide | Nickel (II) oxide | mercury (I) oxide | |
ammonium chloride | iron (III) chloride | Nickel (II) chloride | mercury (I) chloride |
Criss-cross method is used to find the formula and name of compound by balancing the charges.
Chapter 4 Solutions
World of Chemistry, 3rd edition
- Please draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFirefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forward
- TRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forwardRelative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forwardCalculate the pH of 0.0025 M phenol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





