
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
9th Edition
ISBN: 9781305671874
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3.SE, Problem 49AP
Interpretation Introduction
Interpretation:
The structure of malic acid is to be drawn.
Concept introduction:
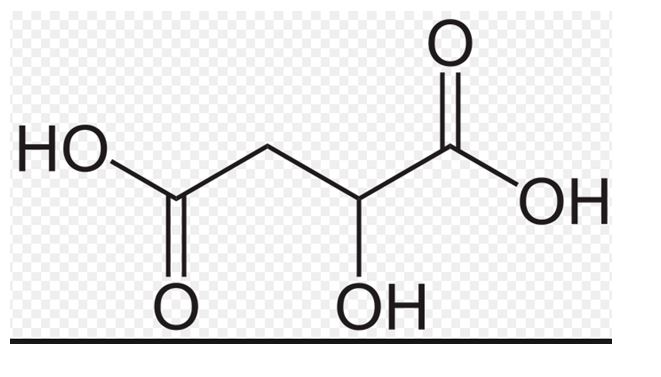
It is a compound present in apple which contributes to the sour taste of it and used as a food additives.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
>
You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other
major side products:
1. ☑
CI
2. H3O+
O
Draw the missing reagent X you think will make this synthesis work in the drawing area below.
If there is no reagent that will make your desired product in good yield or without complications, just check the box under the
drawing area and leave it blank.
Click and drag to start drawing a
structure.
Explanation
Check
?
DO
18
Ar
B
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Don't use ai to answer I will report you answer
Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence point
Chapter 3 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
Ch. 3.1 - Prob. 1PCh. 3.1 - Prob. 2PCh. 3.1 - Identify the functional groups in the following...Ch. 3.2 - Draw structures of the five isomers of C6H14.Ch. 3.2 - Propose structures that meet the following...Ch. 3.2 - Prob. 6PCh. 3.3 - Draw the eight 5-carbon alkyl groups (pentyl...Ch. 3.3 - Identify the carbon atoms in the following...Ch. 3.3 - Prob. 9PCh. 3.3 - Prob. 10P
Ch. 3.4 - Give IUPAC names for the following compounds:Ch. 3.4 - Prob. 12PCh. 3.4 - Name the eight 5-carbon alkyl groups you drew in...Ch. 3.4 - Give the IUPAC name for the following hydrocarbon,...Ch. 3.7 - Make a graph of potential energy versus angle of...Ch. 3.7 - Sight along the C2-C1 bond of 2-methylpropane...Ch. 3.7 - Sight along the C2-C3 bond of 2,3-dimethylbutane,...Ch. 3.7 - Draw a Newman projection along the C2-C3 bond of...Ch. 3.SE - Prob. 19VCCh. 3.SE - Prob. 20VCCh. 3.SE - Draw a Newman projection along the C2-C3 bond of...Ch. 3.SE - Prob. 22APCh. 3.SE - Prob. 23APCh. 3.SE - Propose structures for the following: (a) A...Ch. 3.SE - Prob. 25APCh. 3.SE - Draw the structures of the following molecules:...Ch. 3.SE - Draw structures that meet the following...Ch. 3.SE - Prob. 28APCh. 3.SE - In each of the following sets, which structures...Ch. 3.SE - There are seven constitutional isomers with the...Ch. 3.SE - Prob. 31APCh. 3.SE - Draw compounds that contain the following: (a) A...Ch. 3.SE - Prob. 33APCh. 3.SE - Draw and name all monochloro derivatives of...Ch. 3.SE - Draw structures for the following: (a)...Ch. 3.SE - Prob. 36APCh. 3.SE - Draw a compound that: (a) Has nine primary...Ch. 3.SE - Give IUPAC names for the following compounds:Ch. 3.SE - Name the five isomers of C6H14.Ch. 3.SE - Explain why each of the following names is...Ch. 3.SE - Prob. 41APCh. 3.SE - Consider 2-methylbutane (isopentane). Sighting...Ch. 3.SE - What are the relative energies of the three...Ch. 3.SE - Construct a qualitative potential-energy diagram...Ch. 3.SE - Prob. 45APCh. 3.SE - Draw the most stable conformation of pentane,...Ch. 3.SE - Draw the most stable conformation of...Ch. 3.SE - Prob. 48APCh. 3.SE - Prob. 49APCh. 3.SE - Formaldehyde, H2C=O, is known to all biologists...Ch. 3.SE - Prob. 51APCh. 3.SE - Increased substitution around a bond leads to...Ch. 3.SE - Prob. 53APCh. 3.SE - In the next chapter we'll look at...Ch. 3.SE - We’ll see in the next chapter that there are two...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY