
BIOLOGY 2E
2nd Edition
ISBN: 9781506699851
Author: OpenStax
Publisher: XANEDU PUBLISHING
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 39, Problem 3VCQ
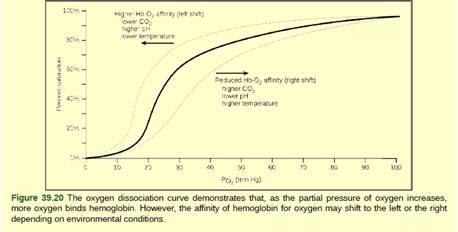
Figure 39.20 The kidneys are responsible for removing excess H+ ions from the blood. If the kidneys fail, what would happen to blood pH and to hemoglobin affinity for oxygen?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Different species or organisms research for ecology
What is the result of the following gram stain:
positive
○ capsulated
○ acid-fast
○ negative
What
type
of stain is the image below:
capsule stain
endospore stain
gram stain
negative stain
ASM MicrobeLibrary.org Keplinger
Chapter 39 Solutions
BIOLOGY 2E
Ch. 39 - Figure 39.7 Which of the following statements...Ch. 39 - Figure 39.13 Which of the following statements is...Ch. 39 - Figure 39.20 The kidneys are responsible for...Ch. 39 - The respiratory system. provides body tissues with...Ch. 39 - Air is warmed and humidified in the nasal...Ch. 39 - Which is the order of airflow during inhalation?...Ch. 39 - The inspiratory reserve volume measures the...Ch. 39 - Of the following, which does not explain why the...Ch. 39 - The total lung capacity is calculated using which...Ch. 39 - How would paralysis of the diaphragm alter...
Ch. 39 - Restrictive airway diseases. increase the...Ch. 39 - Alveolar ventilation remains constant when...Ch. 39 - Which of the following will NOT facilitate the...Ch. 39 - The majority of carbon dioxide in the blood is...Ch. 39 - The majority of oxygen in the blood is transported...Ch. 39 - Describe the function of these terms and describe...Ch. 39 - How does the structure of alveoli maximize gas...Ch. 39 - What does FEV1/FVC measure? What factors may...Ch. 39 - What is the reason for having residual volume in...Ch. 39 - How can a decrease in the percent of oxygen in the...Ch. 39 - If a patient has increased resistance in his or...Ch. 39 - How would increased airway resistance affect...Ch. 39 - Explain how a puncture to the thoracic cavity...Ch. 39 - When someone is standing, gravity stretches the...Ch. 39 - What would happen if no carbonic anhydrase were...Ch. 39 - How does the administration of 100 percent oxygen...
Additional Science Textbook Solutions
Find more solutions based on key concepts
With the initial appearance of the feature we call Now Solve This, a short introduction is in order. The featur...
Concepts of Genetics (12th Edition)
Your bore cells, muscle cells, and skin cells look different because a. different kinds of genes are present in...
Campbell Essential Biology (7th Edition)
What two components contribute to species diversity? Explain how two communities with the same number of specie...
Campbell Biology (11th Edition)
Which type of cartilage is most plentiful in the adult body?
Anatomy & Physiology (6th Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
When working on barley plants, two researchers independently identify a short-plant mutation and develop homozy...
Genetic Analysis: An Integrated Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- What is the result of the acid-fast stain below: Stock Images by Getty Images by Getty Images by Getty Images by Getty Image Getty Images St Soy Getty Images by Getty Images by Getty Images Joy Getty encapsulated O endosporulating negative ○ positivearrow_forwardYou have a stock vial of diligence 75mg in 3ml and need to draw up a dose of 50mg for your patient.how many mls should you draw up to give this dosearrow_forwardYou are recquired to administer 150mg hydrocortisone intravenously,how many mls should you give?(stock =hydrocortisone 100mg in 2mls)arrow_forward
- If someone was working with a 50 MBq F-18 source, what would be the internal and external dose consequences?arrow_forwardWe will be starting a group project next week where you and your group will research and ultimately present on a current research article related to the biology of a pathogen that infects humans. The article could be about the pathogen itself, the disease process related to the pathogen, the immune response to the pathogen, vaccines or treatments that affect the pathogen, or other biology-related study about the pathogen. I recommend that you choose a pathogen that is currently interesting to researchers, so that you will be able to find plenty of articles about it. Avoid choosing a historical disease that no longer circulates. List 3 possible pathogens or diseases that you might want to do for your group project.arrow_forwardnot use ai pleasearrow_forward
- DNK dagi nukleotidlar va undan sintezlangan oqsildagi peptid boglar farqi 901 taga teng bo'lib undagi A jami H boglardan 6,5 marta kam bo'lsa DNK dagi jami H bog‘lar sonini topingarrow_forwardOne of the ways for a cell to generate ATP is through the oxidative phosphorylation. In oxidative phosphorylation 3 ATP are produced from every one NADH molecule. In respiration, every glucose molecule produces 10 NADH molecules. If a cell is growing on 5 glucose molecules, how much ATP can be produced using oxidative phosphorylation/aerobic respiration?arrow_forwardIf a cell is growing on 5 glucose molecules, how much ATP can be produced using oxidative phosphorylation/aerobic respiration?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax


Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning


Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning
Haematology - Red Blood Cell Life Cycle; Author: Armando Hasudungan;https://www.youtube.com/watch?v=cATQFej6oAc;License: Standard youtube license