
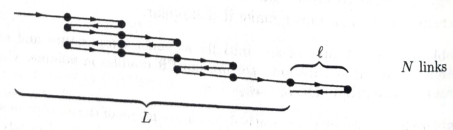
(a) Find an expression for the entropy of this system in term of N and
(b) Write down a formula for L in terms of N and N1,
(c) For a one-dimensional system such as this, the length L is analogous to the volume V of a three-dimensional system. Similarly, the pressure P is replaced by the tension force F. Taking F to be positive when the rubber band is pulling inward, write down and explain the appropriate
(d) Using the thermodynamic Identity, you can flow express the tension force F in terms of a partial derivative of the entropy. From this expression, compute the tension in terms of L, T, N, and
(e) Show that when
(f) Discuss the dependence of the tension force on temperature. If you increase the temperature of a rubber band, does it tend to expand or contract? Does this behavior make sense?
(g) Suppose that you hold a relaxed rubber band in both hands and suddenly stretch it. Would you expect its temperature to increase or decrease? Explain. Test your prediction with a real rubber band (preferably a fairly heavy one with lots of stretch), using your lips or forehead as a thermometer. (Hint: The entropy you computed in part (a) is not the total entropy of the rubber band. There is additional entropy a8sociated with the vibrational energy of the molecules; this entropy depends on U but is approximately independent of L.)
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
An Introduction to Thermal Physics
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Applications and Investigations in Earth Science (9th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Campbell Biology in Focus (2nd Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Two tempered-steel bars, each 16 in. thick, are bonded to a ½ -in. mild-steel bar. This composite bar is subjected as shown to a centric axial load of magnitude P. Both steels are elastoplastic with E= 29 × 106 psi and with yield strengths equal to 100 ksi and 50 ksi, respectively, for the tempered and mild steel. The load P is gradually increased from zero until the deformation of the bar reaches a maximum value dm = 0.04 in. and then decreased back to zero. Take L = 15 in. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. 2.0 in. in. 3 in. 3 16 in. Determine the maximum stress in the tempered-steel bars. The maximum stress in the tempered-steel bars is ksi.arrow_forwardAmmonia enters the compressor of an industrial refrigeration plant at 2 bar, -10°C with a mass flow rate of 15 kg/min and is compressed to 12 bar, 140°C. Heat transfer from the compressor to its surroundings occurs at a rate of 6 kW. For steady-state operation, calculate, (a) the power input to the compressor, in kW, Answer (b) the entropy production rate, in kW/K, for a control volume encompassing the compressor and its immediate surroundings such that heat transfer occurs at 300 K.arrow_forwardNo chatgpt pls will upvotearrow_forward
- Shown to the right is a block of mass m=5.71kgm=5.71kg on a ramp that makes an angle θ=24.1∘θ=24.1∘ with the horizontal. This block is being pushed by a horizontal force, F=229NF=229N. The coefficient of kinetic friction between the two surfaces is μ=0.51μ=0.51. Enter an expression for the acceleration of the block up the ramp using variables from the problem statement together with gg for the acceleration due to gravity. a=arrow_forwardIf the density and atomic mass of copper are respectively 8.80 x 103 kg/m³ and 63.5 kg/kmol (note that 1 kmol = 1,000 mol), and copper has one free electron per copper atom, determine the following. (a) the drift speed of the electrons in a 10 gauge copper wire (2.588 mm in diameter) carrying a 13.5 A current 1.988-4 See if you can obtain an expression for the drift speed of electrons in a copper wire in terms of the current in the wire, the diameter of the wire, the molecular weight and mass density of copper, Avogadro's number, and the charge on an electron. m/s (b) the Hall voltage if a 2.68 T field is applied perpendicular to the wire 3.34e-6 x Can you start with basic equations for the electric and magnetic forces acting on the electrons moving through the wire and obtain a relationship between the magnitude of the electric and magnetic field and the drift speed of the electrons? How is the magnitude of the electric field related to the Hall voltage and the diameter of the wire? Varrow_forward(a) At what speed (in m/s) will a proton move in a circular path of the same radius as an electron that travels at 7.85 x 100 m/s perpendicular to the Earth's magnetic field at an altitude where the field strength is 1.20 x 10-5 T? 4.27e3 m/s (b) What would the radius (in m) of the path be if the proton had the same speed as the electron? 0.685 x m (c) What would the radius (in m) be if the proton had the same kinetic energy as the electron? 0.0084 m (d) What would the radius (in m) be if the proton had the same momentum as the electron? 0.0303 x marrow_forward
- Two charges are placed on the x axis. One of the charges (91 = +6.63 μC) is at x₁ = +3.00 cm and the other (92 = -24.2 μC) is at x2 = +9.00 cm. Find the net electric field (magnitude and direction given as a plus or minus sign) at (a) x = 0 cm and (b) x = +6.00 cm.arrow_forwardThe diagram shows the all of the forces acting on a body of mass 2.76 kg. The three forces have magnitudes F1 = 65.2 N, F2 = 21.6 N, and F3 = 77.9 N, with directions as indicted in the diagram, where θ = 49.9 degrees and φ = 21.1 degrees. The dashed lines are parallel to the x and y axes. At t = 0, the body is moving at a speed of 6.87 m/s in the positive x direction. a. whats the x component of the acceleration? b. whats the y component of the acceleration? c. whats the speed of the body in m/s at t = 12.3s? d. whats the magnitude of the displacement of the body n meters between t = 0 and 12.3s?arrow_forwardNo chatgpt pls will upvotearrow_forward
- No chatgpt pls will upvotearrow_forwardA cylinder with a piston contains 0.153 mol of nitrogen at a pressure of 1.83×105 Pa and a temperature of 290 K. The nitrogen may be treated as an ideal gas. The gas is first compressed isobarically to half its original volume. It then expands adiabatically back to its original volume, and finally it is heated isochorically to its original pressure. Part A Compute the temperature at the beginning of the adiabatic expansion. Express your answer in kelvins. ΕΠΙ ΑΣΦ T₁ = ? K Submit Request Answer Part B Compute the temperature at the end of the adiabatic expansion. Express your answer in kelvins. Π ΑΣΦ T₂ = Submit Request Answer Part C Compute the minimum pressure. Express your answer in pascals. ΕΠΙ ΑΣΦ P = Submit Request Answer ? ? K Paarrow_forwardLearning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation pV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and T is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, pV = constant. Τ One can see that, if the amount of gas remains constant, it is impossible to change just one parameter of the gas: At least one more parameter would also change. For instance, if the pressure of the gas is changed, we can be sure that either the volume or the temperature of the gas (or, maybe, both!) would also change. To explore these changes, it is often convenient to draw a graph showing one parameter as a function of the other. Although there are many choices of axes, the most common one is a plot of pressure as a function of volume: a pV diagram. In this problem, you…arrow_forward
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning





