
Concept explainers
Although styrene undergoes both cationic and anionic
a. 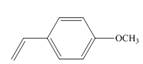 c.
c. 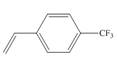
b. 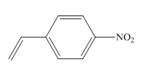 d.
d. 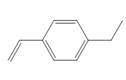
(a)
Interpretation: The method, anionic or cationic polymerization preferred with the given substituted styrene is to be identified, and an explanation corresponding to it is to be stated.
Concept introduction: An alkene
Answer to Problem 30.43P
The given substituted styrene prefers to undergo cationic polymerization, due to presence of electron releasing group on benzene ring.
Explanation of Solution
The given substituted styrene resembles to
An alkene
An electron releasing group releases electron density towards benzene ring and stabilizes carbocation formed during the cationic polymerization, whereas an electron withdrawing group withdraws electron density towards itself and stabilizes carbanion formed during the anionic polymerization.
Since the given substituted styrene involves bonding of an electron releasing group to benzene ring, it prefers to undergo cationic polymerization.
(b)
Interpretation:The method, anionic or cationic polymerization preferred with the given substituted styrene is to be identified, and an explanation corresponding to it is to be stated.
Concept introduction: An alkene
Answer to Problem 30.43P
The given substituted styrene prefers to undergo anionic polymerization, due to presence of electron withdrawing group on benzene ring.
Explanation of Solution
The given substituted styrene resembles to
An alkene
An electron releasing group releases electron density towards benzene ring and stabilizes carbocation formed during the cationic polymerization, whereas an electron withdrawing group withdraws electron density towards itself and stabilizes carbanion formed during the anionic polymerization.
Since the given substituted styrene involves bonding of an electron withdrawing group to benzene ring, it prefers to undergo anionic polymerization.
(c)
Interpretation:The method, anionic or cationic polymerization preferred with the given substituted styrene is to be identified, and an explanation corresponding to it is to be stated.
Concept introduction: An alkene
Answer to Problem 30.43P
The given substituted styrene prefers to undergo anionic polymerization, due to presence of electron withdrawing group on benzene ring.
Explanation of Solution
The given substituted styrene resembles to
An alkene
An electron releasing group releases electron density towards benzene ring and stabilizes carbocation formed during the cationic polymerization, whereas an electron withdrawing group withdraws electron density towards itself and stabilizes carbanion formed during the anionic polymerization.
Since the given substituted styrene involves bonding of an electron withdrawing group to benzene ring, it prefers to undergo anionic polymerization.
(d)
Interpretation:The method, anionic or cationic polymerization preferred with the given substituted styrene is to be identified, and an explanation corresponding to it is to be stated.
Concept introduction: An alkene
Answer to Problem 30.43P
The given substituted styrene prefers to undergo cationic polymerization, due to presence of electron releasing group on benzene ring.
Explanation of Solution
The given substituted styrene resembles to
An alkene
An electron releasing group releases electron density towards benzene ring and stabilizes carbocation formed during the cationic polymerization, whereas an electron withdrawing group withdraws electron density towards itself and stabilizes carbanion formed during the anionic polymerization.
Since the given substituted styrene involves bonding of an electron releasing group to benzene ring, it prefers to undergo cationic polymerization.
(a) The given substituted styrene prefers to undergo cationic polymerization, due to presence of an electron releasing group on benzene ring.
(b) The given substituted styrene prefers to undergo anionic polymerization, due to presence of an electron withdrawing group on benzene ring.
(c) The given substituted styrene prefers to undergo anionic polymerization, due to presence of an electron withdrawing group on benzene ring.
(d) The given substituted styrene prefers to undergo cationic polymerization, due to presence of an electron releasing group on benzene ring.
Want to see more full solutions like this?
Chapter 30 Solutions
Organic Chemistry
- Br HO ? HO ✓ OHarrow_forwardUse the literature Ka value of the acetic acid, and the data below to answer these questions. Note: You will not use the experimental titration graphs to answer the questions that follow. Group #1: Buffer pH = 4.35 Group #2: Buffer pH = 4.70 Group #3: Buffer pH = 5.00 Group #4: Buffer pH = 5.30 Use the Henderson-Hasselbalch equation, the buffer pH provided and the literature pKa value of acetic acid to perform the following: a) calculate the ratios of [acetate]/[acetic acid] for each of the 4 groups buffer solutions above. b) using the calculated ratios, which group solution will provide the best optimal buffer (Hint: what [acetate]/[acetic acid] ratio value is expected for an optimal buffer?) c) explain your choicearrow_forwardHow would you prepare 1 liter of a 50 mM Phosphate buffer at pH 7.5 beginning with K3PO4 and 1 M HCl or 1 M NaOH? Please help and show calculations. Thank youarrow_forward
- Draw the four most importantcontributing structures of the cation intermediate thatforms in the electrophilic chlorination of phenol,(C6H5OH) to form p-chlorophenol. Put a circle aroundthe best one. Can you please each step and also how you would approach a similar problem. Thank you!arrow_forwardA 100mM lactic acid/lactate buffer was found to have a lactate to lactic acid ratio of 2 and a pH of 4.2. What is the pKa of lactic acid? Can you please help show the calculations?arrow_forwardUsing line angle formulas, draw thestructures of and name four alkanes that have total of 7carbons, one of which is tertiary.Please explain this in detail and can you also explain how to approach a similar problem like this as well?arrow_forward
- Using dashed line wedge projections drawthe indicated compounds and indicate whether thecompound you have drawn is R or S.(a) The two enantiomers of 2-chlorobutane. Can you please explain your steps and how you would approach a similar problem. Thank you!arrow_forward5) There are no lone pairs shown in the structure below. Please add in all lone pairs and then give the hybridization scheme for the compound. (8) 10,11 7) 1.2.3 H 4 | 14 8) COC 12 13 H 16 15 H7 9) - 5.6 C 8 H 10) H 1). 2) 3)_ 11) 12) 13) 4)_ 14) 5) 15) 16) 6)arrow_forwardThe sum of the numbers in the name of isA. 11; B. 13; C. 10; D. 12; E. none of the other answers iscorrect. I believe the awnser should be E to this problem but the solution to this problem is D 12. I'm honestly unsure how that's the solution. If you can please explain the steps to this type of problem and how to approach a problem like this it would be greatly appreciated!arrow_forward
- Consider the following data for phosphorus: g atomic mass 30.974 mol electronegativity 2.19 kJ electron affinity 72. mol kJ ionization energy 1011.8 mol kJ heat of fusion 0.64 mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? 2+ + (1) P (g) + e → P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): Does the following reaction absorb or release energy? 00 release absorb Can't be decided with the data given. yes no ☐ kJ/mol (²) P* (8) + + + e →>> P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (2) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (2): ☐ release absorb Can't be decided with the data given. yes no kJ/mol аarrow_forwardThe number of hydrogens in an alkyne that has a main chain of 14carbons to which are attached a cyclobutyl ring, a benzene ring, an–OH group, and a Br is A. 34; B. 35; C. 36; D. 24; E. 43arrow_forwardHello! I have a 500 Hz H-NMR for 1,5-bis-(4-methoxyphenyl)-penta-1,4-dien-3-one. I need to label the signals with the corresponding H's. Then, find out if the two alkenes are cis or trans by calculating the J values. I believe that I have the H-NMR labeled correctly, but not sure if I got the J values correct to determine if the two alkenes in the compound will make the compound cis or trans.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning  Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




