
Concept explainers
Classify each terpene and terpenoid in Problem 30.28 (e.g., as a monoterpene, sesquiterpene, etc.).
(a)
Interpretation: The terpene and terpenoid are to be classified as monoterpene, sesquiterpene, etc.
Concept introduction: Terpenes are naturally occurring compounds that are present in plants and animals. Terpenes contain one or more isoprene units. Terpenoids are derivatives of terpenes with oxygen containing functional group such as carbonyl groups. Monoterpenes contain two isoprene units, sesquiterpene contain three isoprene units etc.
Answer to Problem 30.29P
Neral is classified as monoterpene.
Explanation of Solution
The structure of isoprene is,
Figure 1
The isoprene unit contains four carbon atoms in the long chain and one carbon atom in branch. Isoprene unit in a terpene consists of carbon-carbon sigma or pi bonds.
The isoprene units in the given compound are shown below.
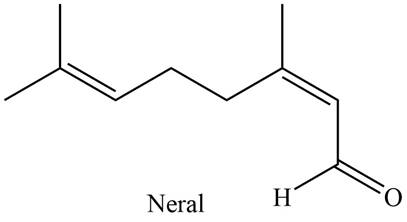
Figure 2
The highlighted bonds represent the isoprene unit. There are two isoprene units present in the given compound. Neral is classified as monoterpene.
Neral is classified as monoterpene.
(b)
Interpretation: The terpene and terpenoid are to be classified as monoterpene, sesquiterpene, etc.
Concept introduction: Terpenes are naturally occurring compounds that are present in plants and animals. Terpenes contain one or more isoprene units. Terpenoids are derivatives of terpenes with oxygen containing functional group such as carbonyl groups. Monoterpenes contain two isoprene units, sesquiterpene contain three isoprene units etc.
Answer to Problem 30.29P
Carvone is classified as monoterpene.
Explanation of Solution
The structure of isoprene is,
Figure 1
The isoprene unit contains four carbon atoms in the long chain and one carbon atom in branch. Isoprene unit in a terpene consists of carbon-carbon sigma or pi bonds.
The isoprene units in the given compound are shown below.
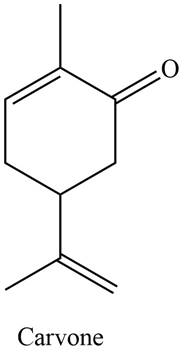
Figure 3
The highlighted bonds represent the isoprene unit. There are two isoprene units present in the given compound. Carvone is classified as monoterpene.
Carvone is classified as monoterpene.
(c)
Interpretation: The terpene and terpenoid are to be classified as monoterpene, sesquiterpene, etc.
Concept introduction: Terpenes are naturally occurring compounds that are present in plants and animals. Terpenes contain one or more isoprene units. Terpenoids are derivatives of terpenes with oxygen containing functional group such as carbonyl groups. Monoterpenes contain two isoprene units, sesquiterpene contain three isoprene units etc.
Answer to Problem 30.29P
Lycopene is classified as tetraterpene.
Explanation of Solution
The structure of isoprene is,
Figure 1
The isoprene unit contains four carbon atoms in the long chain and one carbon atom in branch. Isoprene unit in a terpene consists of carbon-carbon sigma or pi bonds.
The isoprene units in the given compound are shown below.
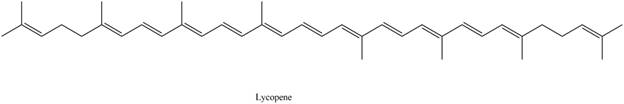
Figure 4
The highlighted bonds represent the isoprene unit. There are eight isoprene units present in the given compound. Lycopene is classified as tetraterpene.
Lycopene is classified as tetraterpene.
(d)
Interpretation: The terpene and terpenoid are to be classified as monoterpene, sesquiterpene, etc.
Concept introduction: Terpenes are naturally occurring compounds that are present in plants and animals. Terpenes contain one or more isoprene units. Terpenoids are derivatives of terpenes with oxygen containing functional group such as carbonyl groups. Monoterpenes contain two isoprene units, sesquiterpene contain three isoprene units etc.
Answer to Problem 30.29P
The
Explanation of Solution
The structure of isoprene is,

Figure 1
The isoprene unit contains four carbon atoms in the long chain and one carbon atom in branch. Isoprene unit in a terpene consists of carbon-carbon sigma or pi bonds.
The isoprene units in the given compound are shown below.
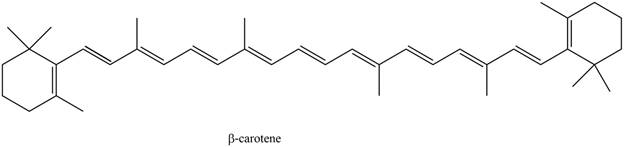
Figure 5
The highlighted bonds represent the isoprene unit. There are eight isoprene units present in the given compound. The
The
(e)
Interpretation: The terpene and terpenoid are to be classified as monoterpene, sesquiterpene, etc.
Concept introduction: Terpenes are naturally occurring compounds that are present in plants and animals. Terpenes contain one or more isoprene units. Terpenoids are derivatives of terpenes with oxygen containing functional group such as carbonyl groups. Monoterpenes contain two isoprene units, sesquiterpene contain three isoprene units etc.
Answer to Problem 30.29P
Patchouli alcohol is classified as sesquiterpene.
Explanation of Solution
The structure of isoprene is,

Figure 1
The isoprene unit contains four carbon atoms in the long chain and one carbon atom in branch. Isoprene unit in a terpene consists of carbon-carbon sigma or pi bonds.
The isoprene units in the given compound are shown below.
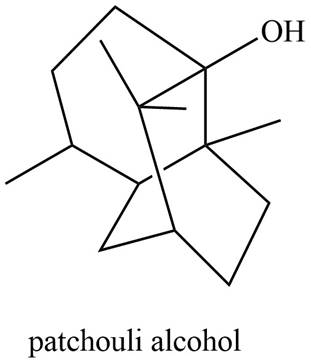
Figure 6
The highlighted bonds represent the isoprene unit. There are three isoprene units present in the given compound. Patchouli alcohol is classified as sesquiterpene.
Patchouli alcohol is classified as sesquiterpene.
(f)
Interpretation: The terpene and terpenoid are to be classified as monoterpene, sesquiterpene, etc.
Concept introduction: Terpenes are naturally occurring compounds that are present in plants and animals. Terpenes contain one or more isoprene units. Terpenoids are derivatives of terpenes with oxygen containing functional group such as carbonyl groups. Monoterpenes contain two isoprene units, sesquiterpene contain three isoprene units etc.
Answer to Problem 30.29P
Periplanone B is classified as sesquiterpene.
Explanation of Solution
The structure of isoprene is,
Figure 1
The isoprene unit contains four carbon atoms in the long chain and one carbon atom in branch. Isoprene unit in a terpene consists of carbon-carbon sigma or pi bonds.
The isoprene units in the given compound are shown below.
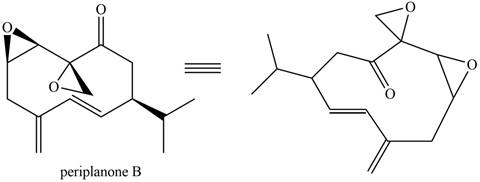
Figure 7
The highlighted bonds represent the isoprene unit. There are three isoprene units present in the given compound. Periplanone B is classified as sesquiterpene.
Periplanone B is classified as sesquiterpene.
(g)
Interpretation: The terpene and terpenoid are to be classified as monoterpene, sesquiterpene, etc.
Concept introduction: Terpenes are naturally occurring compounds that are present in plants and animals. Terpenes contain one or more isoprene units. Terpenoids are derivatives of terpenes with oxygen containing functional group such as carbonyl groups. Monoterpenes contain two isoprene units, sesquiterpene contain three isoprene units etc.
Answer to Problem 30.29P
Dextropimaric acid is classified as diterpene.
Explanation of Solution
The structure of isoprene is,

Figure 1
The isoprene unit contains four carbon atoms in the long chain and one carbon atom in branch. Isoprene unit in a terpene consists of carbon-carbon sigma or pi bonds.
The isoprene units in the given compound are shown below.
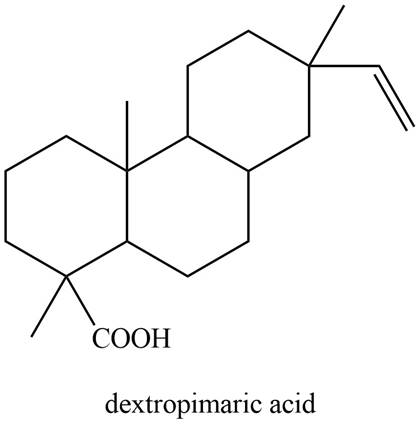
Figure 8
The highlighted bonds represent the isoprene unit. There are four isoprene units present in the given compound. Dextropimaric acid is classified as diterpene.
Dextropimaric acid is classified as diterpene.
(h)
Interpretation: The terpene and terpenoid are to be classified as monoterpene, sesquiterpene, etc.
Concept introduction: Terpenes are naturally occurring compounds that are present in plants and animals. Terpenes contain one or more isoprene units. Terpenoids are derivatives of terpenes with oxygen containing functional group such as carbonyl groups. Monoterpenes contain two isoprene units, sesquiterpene contain three isoprene units etc.
Answer to Problem 30.29P
The
Explanation of Solution
The structure of isoprene is,
Figure 1
The isoprene unit contains four carbon atoms in the long chain and one carbon atom in branch. Isoprene unit in a terpene consists of carbon-carbon sigma or pi bonds.
The isoprene units in the given compound are shown below.
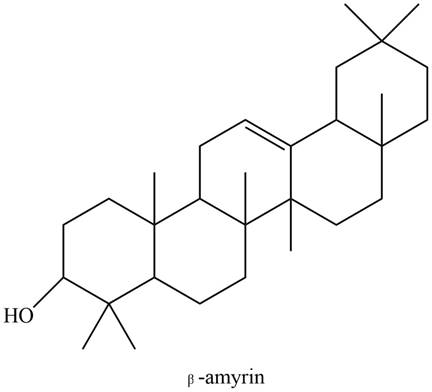
Figure 9
The highlighted bonds represent the isoprene unit. There are four isoprene units present in the given compound. The
The
Want to see more full solutions like this?
Chapter 30 Solutions
Organic Chemistry
- Differentiate between plastic deformation, elastic deformation, viscoelastic deformation and viscoplastic deformation.arrow_forward1.57 Draw all reasonable resonance structures for the following cation. Then draw the resonance hybrid.arrow_forwardFor the two questions below, draw the mechanism and form the major product.arrow_forward
- Indicate similarities and differences between natural, exchanged and pillared clays.arrow_forwardShow work. don't give Ai generated solutionarrow_forwardIn intercalation compounds, their sheets can be neutral or have a negative or positive charge, depending on the nature of the incorporated species and its structure. Is this statement correct?arrow_forward
- This thermodynamic cycle describes the formation of an ionic compound MX2 from a metal element M and nonmetal element X in their standard states. What is the lattice enthalpy of MX2 ? What is the enthalpy formation of MX2 ? Suppose both the heat of sublimation of M and the ionization enthalpy of M were smaller. Would MX2 be more stable? Or less? or impossible to tell without more information?arrow_forward7. Draw the mechanism to describe the following transformation: Note: This is a base catalyzed reaction. So, the last steps must make [OH]- OH [OH]¯ OH Heat Oarrow_forwardShow work with explanation...don't give Ai generated solutionarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
