
(a)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 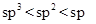 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(b)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 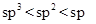 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(c)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 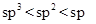 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(d)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 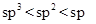 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(e)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 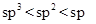 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(f)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 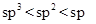 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(g)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 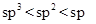 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
(h)
Interpretation:
The more acidic compound from each given pair of compounds should be identified in each of the given anions.
Concept introduction:
Acidic strength in the molecule depends on the stability of the Conjugate anion.
The anion involving in resonance in a molecule will have greater stability when compared to molecules having single anion. In resonance the delocalization of anion would take place.
In a molecule where the anion is carried by a more electronegative atom will show more stability. The greater electronegativity makes anion closer to the nucleus hence more stabilized. If the negative charge located atom is larger enough to hold the negative charge, then anion attains more stability.
The carbanion stability varies with the percentage of ‘s’character in its hybridization state. Since the s-orbital is closer to nucleus than p-orbital, the hybridized orbital having more‘s’ character will have more stability to accommodate anion (due to high nuclear charge). The order is 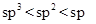 .
.
Inductive effect in a compound will affect the anionic stability. +I groups in the compound stabilizes the negative charge of anion.
To find: the more acidic compound from each given pair of compounds.
Trending nowThis is a popular solution!

Chapter 3 Solutions
ORGANIC CHEMISTRY,SOLNS...-ETEXT+BOX
- Please help me with number 1-3. Thank you so much.arrow_forwardDraw the major product of this reaction ingnore the inorganic byproducts. 1. NaOCH2CH3 at 25 C 2. PhCH2Br (1 eq)arrow_forwardAt 90ºC the vapor pressure of ortho-xylene is 20 kPa and that of meta-xylene is 18 kPa. What is the composition of the vapor in equilibrium with a mixture in which the mole fraction of o-xylene is 0.60?arrow_forward
- Draw the products of this reduction of a ketone with sodium borohydride. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, where applicableIgnore any inorganic byproducts. 1) NaBH4 2) HCI/H2O Select to Drawarrow_forwardWhy do you think people who live at high altitudes are advised to add salt to water when boiling food like pasta? What mole fraction of NaCl is needed to raise the boiling point of H2O by 3˚C? Does the amount of salt added to water (typically about one teaspoon to four quarts of water) substantially change the boiling point? (Kb (H2O) = 0.51˚C/molal.)arrow_forwardpls help asaparrow_forward
- pls help asaparrow_forward9. Consider the following galvanic cell: Fe (s) | Fe(NO3)2 (aq) || Sn(NO3)2 (aq) | Sn (s) a. Write an equation for the half reactions occurring at the anode and cathode. b. Calculate the standard cell potential Show all of your work. c. Draw and label the galvanic cell, including the anode and cathode, direction of electron flow, and direction of ion migration.arrow_forwardpls help asaparrow_forward
- 11. Use the equation below to answer the following questions: 2 Al(s) + 3 Cd(NO3)2 (aq) → 2 Al(NO3)3 (aq) + 3 Cd(s) a. What is the net ionic equation for the reaction? b. Which species is a spectator ion in this reaction? Define a spectator ion. c. Identify the oxidizing agent and the reducing agent.arrow_forwardpls help asaparrow_forwardpls help asaparrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





