
Concept explainers
(a)
Interpretation:
Molecular formula for unknown compound has to be determined.
Concept Introduction:
Here,
(a)
Answer to Problem 3.9E
Mass of
Explanation of Solution
Conversion factor to convert
Rearrange equation (1) to determine value of
Substitute
Number of moles of nitrogen present in
Number of moles of hydrogen gas present in
Ratio of nitrogen to hydrogen can be calculated as follows:
Therefore formula unit is
Total molar mass of gas can be calculated as follows:
Therefore, number of formula unit of
(b)
Interpretation:
Lewis structure of
Concept Introduction:
Lewis structure represents covalent bonds and describes valence electrons configuration of atoms. The covalent bonds are depicted by lines and unshared electron pairs by pairs of dots. The sequence to write Lewis structure of some molecule is given as follows:
- The central atom is identified and various other atoms are arranged around it. This central atom so chosen is often the least electronegative.
- Total valence electrons are estimated for each atom.
- single bond is first placed between each atom pair.
- The electrons left can be allocated as unshared electron pairs or as multiple bonds around
symbol of element to satisfy the octet (or duplet) for each atom. - Add charge on overall structure in case of polyatomic cation or anion.
(b)
Explanation of Solution
The molecule
Electronic configuration of
Electronic configuration of
Thus total valence electrons are sum of the valence electrons for each atom in
Skeleton structure
Hence, 4 electrons are allocated as 2 lone pairs on each nitrogen atom. The Lewis structure of
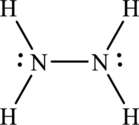
(c)
Interpretation:
Amount of nitrogen gas effused through small hole in
Concept Introduction:
Effusion is explained as movement of the gas molecule through small hole. Diffusion can be explained as one gas molecule mix with another gas molecule by random motion.
(c)
Answer to Problem 3.9E
Amount of nitrogen gas effused through small hole in
Explanation of Solution
The expression for molar masses of
Rearrange equation (3) to calculate amount of
Substitute
Hence amount of nitrogen gas effused through small hole in
Want to see more full solutions like this?
Chapter 3 Solutions
ACHIEVE/CHEMICAL PRINCIPLES ACCESS 2TERM
- If CH3COCH2CH(OCH3)2 (4,4-dimethoxy-2-butanone) and hydrazine react, two isomeric products are formed. State their structure and which will be the majority.arrow_forward+ Reset Provide the correct IUPAC name for the compound shown here. 4-methylhept-2-ene (Z)- (E)- 1-6-5-2-3-4- cyclo iso tert- sec- di tri hept hex oct meth eth pent ane yne ene ylarrow_forward+ Provide the correct IUPAC name for the compound shown here. Reset H3C H H C CH3 CH-CH3 1-3-methylpent ene trans- cis- 5-6-3-1-2-4- tert- tri sec- di cyclo iso but pent hex meth prop eth yl ane ene yne ☑arrow_forward
- drawing, no aiarrow_forwarddrawing, no aiarrow_forwardDraw the major organic product when each of the bellow reagents is added to 3,3-dimethylbutere. ✓ 3rd attempt Part 1 (0.3 point) H.C CH CH + 1. BHG THF 210 NaOH NJ 10000 Part 2 (0.3 point) HC- CH HC 2741 OH a Search 1. He|DA HO 2. NIBH さ 士 Ju See Periodic Table See Hint j = uz C H F F boxarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





