
(a)
Interpretation:
Conjugate acids for the given bases are needed to be drawn.
Concept introduction:
According to Bronsted-Lowry concept, base is a proton acceptor. The protonated base is called conjugate acid. An electron pair is shared from base to the proton.
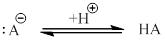
(b)
Interpretation:
Conjugate acids for the given bases are needed to be drawn.
Concept introduction:
According to Bronsted-Lowry concept, base is a proton acceptor. The protonated base is called conjugate acid. An electron pair is shared from base to the proton.
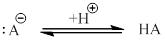
(c)
Interpretation:
Conjugate acids for the given bases are needed to be drawn.
Concept introduction:
According to Bronsted-Lowry concept, base is a proton acceptor. The protonated base is called conjugate acid. An electron pair is shared from base to the proton.
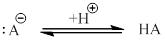
(d)
Interpretation:
Conjugate acids for the given bases are needed to be drawn.
Concept introduction:
According to Bronsted-Lowry concept, base is a proton acceptor. The protonated base is called conjugate acid. An electron pair is shared from base to the proton.
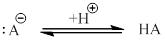
(e)
Interpretation:
Conjugate acids for the given bases are needed to be drawn.
Concept introduction:
According to Bronsted-Lowry concept, base is a proton acceptor. The protonated base is called conjugate acid. An electron pair is shared from base to the proton.
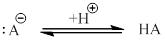
(f)
Interpretation:
Conjugate acids for the given bases are needed to be drawn.
Concept introduction:
According to Bronsted-Lowry concept, base is a proton acceptor. The protonated base is called conjugate acid. An electron pair is shared from base to the proton.
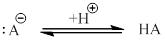
(g)
Interpretation:
Conjugate acids for the given bases are needed to be drawn.
Concept introduction:
According to Bronsted-Lowry concept, base is a proton acceptor. The protonated base is called conjugate acid. An electron pair is shared from base to the proton.
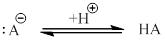
(h)
Interpretation:
Conjugate acids for the given bases are needed to be drawn.
Concept introduction:
According to Bronsted-Lowry concept, base is a proton acceptor. The protonated base is called conjugate acid. An electron pair is shared from base to the proton.
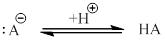
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Given the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





