
Interpretation:
The planar density and packing fraction for the planes (100), (110), and (111) in BCC lithium needs to be calculated and the plane which is close packed needs to be identified.
Concept introduction:
Lithium has a body-centered cubic structure with a lattice parameter of 3.5089
The atomic radius can be calculated as follows:
Here,
Planar density is the ratio of the area of the plane to a number of atoms in a plane.
Answer to Problem 3.77P
Explanation of Solution
For plane (100):
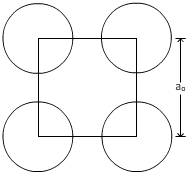
From figure,
For plane (110):
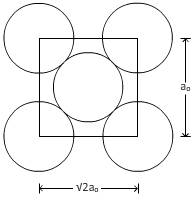
From figure,
For plane(111):
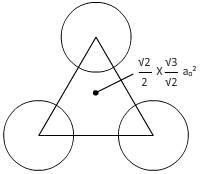
From figure,
Higher the packing fraction, more dense it will be.
From the above calculation, packing fraction of all the planes are:
Hence, plane (110) is highly denser among all the planes because it has a higher value of packing fraction.
Want to see more full solutions like this?
Chapter 3 Solutions
Essentials Of Materials Science And Engineering
- i need help with this question i tried by myself and so i am uploadding the question to be quided with step by step solution and please do not use chat gpt i am trying to learn thank you.arrow_forwardUse dynamic programming to find an LCS of X=[1; 0; 0; 1; 0; 1; 0] and Y=[0; 1; 0; 1; 1; 0]. You need to illustrate your intermediate results by providing the c matrix and showing how you reconstruct the LCS by tracing backwards.arrow_forwardi need help with this question i tried by myself and so i am uploadding the question to be quided with step by step solution and please do not use chat gpt i am trying to learn thank you. i only need help with the second question pleasearrow_forward
- ⚫ your circuit diagrams for your basic bricks, such as AND, OR, XOR gates and 1 bit multiplexers, ⚫ your circuit diagrams for your extended full adder, designed in Section 1 and ⚫ your circuit diagrams for your 8-bit arithmetical-logical unit, designed in Section 2. 1 An Extended Full Adder In this Section, we are going to design an extended full adder circuit (EFA). That EFA takes 6 one bit inputs: aj, bj, Cin, Tin, t₁ and to. Depending on the four possible combinations of values on t₁ and to, the EFA produces 3 one bit outputs: sj, Cout and rout. The EFA can be specified in principle by a truth table with 26 = 64 entries and 3 outputs. However, as the EFA ignores certain inputs in certain cases, it is easier to work with the following overview specification, depending only on t₁ and to in the first place: t₁ to Description 00 Output Relationship Ignored Inputs Addition Mode 2 Coutsjaj + bj + Cin, Tout= 0 Tin 0 1 Shift Left Mode Sj = Cin, Cout=bj, rout = 0 rin, aj 10 1 1 Shift Right…arrow_forwardi need help with this question i tried by myself and so i am uploadding the question to be quided with step by step solution and please do not use chat gpt i am trying to learn thank you. i only need help with the second question pleasearrow_forwardPlease provide explaination and workings.arrow_forward
- Homework#5arrow_forwardA closed-cycle gas turbine unit operating with maximum and minimum temperature of 760oC and 20oC has a pressure ratio of 7/1. Calculate the ideal cycle efficiency and the work ratioarrow_forwardConsider a steam power plant that operates on a simple, ideal Rankine cycle and has a net power output of 45 MW. Steam enters the turbine at 7 MPa and 500°C and is cooled in the condenser at a pressure of 10 kPa by running cooling water from a lake through the tubes of the condenser at a rate of 2000 kg/s. Show the cycle on a T-s diagram with respect to saturation lines, and determine The thermal efficiency of the cycle,The mass flow rate of the steam and the temperature rise of the cooling waterarrow_forwardTwo reversible heat engines operate in series between a source at 600°C, and a sink at 30°C. If the engines have equal efficiencies and the first rejects 400 kJ to the second, calculate: the temperature at which heat is supplied to the second engine, The heat taken from the source; and The work done by each engine. Assume each engine operates on the Carnot cyclearrow_forwardDon't use ai to answer I will report you answer..arrow_forwardA steam turbine operates at steady state with inlet conditions of P1 = 5 bar, T1 = 320°C. Steam leaves the turbine at a pressure of 1 bar. There is no significant heat transfer between the turbine and its surroundings, and kinetic and potential energy changes between inlet and exit are negligible. If the isentropic turbine efficiency is 75%, determine the work developed per unit mass of steam flowing through the turbine, in kJ/kgarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





