
Interpretation:
The table has to be completed by filling in the formula of the ionic compound from the cations and anions.
Explanation of Solution
The cation is
Bromide
Hydroxide
Hydrogen carbonate
Sulfite
Phosphate
The cation is
Bromide
Hydroxide
Hydrogen carbonate
Sulfite
Phosphate
The cation is
Bromide
Hydroxide
Hydrogen carbonate
Sulfite
Phosphate
The figure below shows the complete table by filling in the formula of the ionic compound from the cations and anions is,
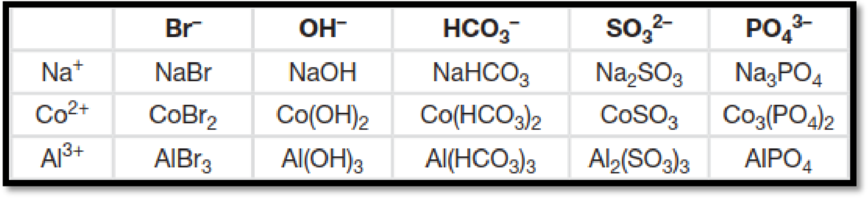
Figure 1
Want to see more full solutions like this?
Chapter 3 Solutions
Principles of General, Organic, Biological Chemistry
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
- Can I please get help with identifying these?arrow_forward4. Calculate the pH of a 0.10 M acetic acid (CH3COOH) solution if the Ka of acetic acid = 1.8 x 10-5arrow_forwardDraw the Zaitsev product of the dehydration of this alcohol. + I X 5 OH ざ~ TSOH Click and drag to start drawing a structure.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





