
Concept explainers
(a)
Interpretation:
An explanation regarding the arrangement of the compounds in order of their decreasing
Concept introduction:
The
Answer to Problem 3.29P
The arrangement of the given compounds in decreasing order of their
Explanation of Solution
The given compounds are shown below.
Figure 1
The acidity of an atom is directly proportional to the electronegativity of an atom. The
Compound C contains
Compound A contains sulfur atom which is less electronegative than oxygen atom but it contains a chlorine atom. Chlorine atom is electronegative in nature due to which acidity of compound A increases.
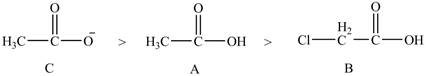
Therefore, the order of acidity of the compounds is shown below.
Figure 2
The
Therefore, the order of decreasing
Figure 3
The order of decreasing
The arrangement of the given compounds in decreasing order of their
(b)
Interpretation:
An explanation regarding the arrangement of the compounds in order of their decreasing
Concept introduction:
The
Answer to Problem 3.29P
The arrangement of the order of decreasing
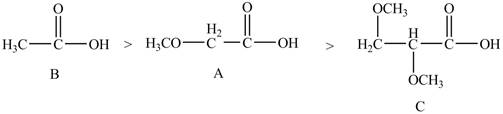
Explanation of Solution
The given compounds are shown below.
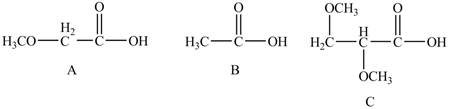
Figure 4
The acidity of an atom is directly proportional to the electronegativity of an atom. Compound C contains two
Compound A contains only one
Compound B contains
Therefore, the order of acidity of the compounds is shown below.
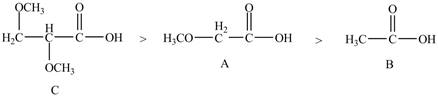
Figure 5
The
Therefore, the order of decreasing
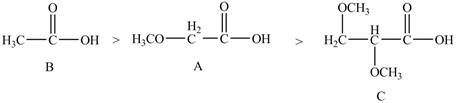
Figure 6
The order of decreasing
The arrangement of the order of decreasing
(c)
Interpretation:
An explanation regarding the arrangement of the compounds in order of their decreasing
Concept introduction:
The
Answer to Problem 3.29P
The arrangement of the order of decreasing
Explanation of Solution
The given compounds are shown below.
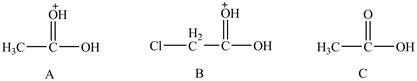
Figure 7
The conjugate bases of the compounds are shown below.
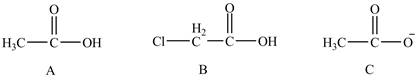
Figure 8
Compound C shown in Figure 8 is more basic than compound A and B due he presence of carboxylate ion. Compound B contains one chlorine atom which is an electron-withdrawing group. Thus, due to
The order of basicity of the compounds is shown below.
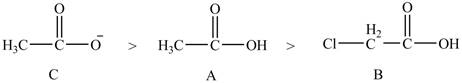
Figure 9
The order of basicity is directly proportional to the
Therefore, the order of decreasing
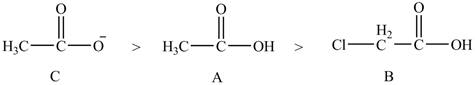
Figure 9
The order of decreasing
The arrangement of the order of decreasing
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry Study Guide and Solutions
- H I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forwardDraw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forward
- Poly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forwardDraw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forward
- Draw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forwardDraw the monomers required to synthesize this condensation polymer please.arrow_forward
- Provide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forwardIdentify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

