
Certain solid substances, known as hydrated compounds, have well-defined molecular ratios of water to some other species. For example, calcium sulfate dihydrate (commonly known as gypsum, CaSO42H2O), has 2 moles of water per mole of calcium sulfate; alternatively, it may be said that 1 mole of gypsum consists of 1 mole of calcium sulfate and 2 moles of water. The water in such substances is called water of hydration. (More information about hydrated salts is given in Chapter 6.)
In order to eliminate the discharge of sulfuric acid into the environment, a process has been developed in which the acid is reacted with aragonite (CaCO3) to produce calcium sulfate. The calcium sulfate then comes out of solution in a crystallizer to form a slurry (a suspension of solid particles in a liquid) of solid gypsum particles suspended in an aqueous CaSO4 solution. The slurry flows front tlte crystallizer to a filter in which the particles arc collected as a filter cake. The filter cake, which is 95.0 wt% solid gypsum and the remainder CaSO4 solution, is fed to a dryer in which all water (including the water of hydration in the crystals) is driven off to yield anhydrous (water-free) CaSO4 as product A flowchart and relevant process data arc given below.
Solids content of slurry leaving crystallizer 0.35 kg CaSO4-2H2O/L slurry CaSO4 content of slurry liquid: 0.209g CaSO4/100g H2O Specific gravities: CaSO4-2H2O(s), 2.32; liquid solutions, 1.05
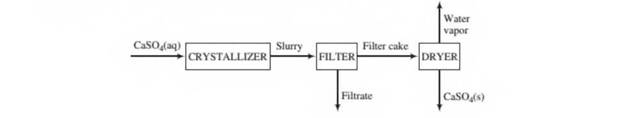
- Briefly explain in your own words the functions of the three units (crystallizer, filter, and dryer).
- Take a basis of one liter of solution leaving the crystallizer and calculate tlte mass (kg) and volume (L) of solid gypsum, the mass of CaSO4 in the gypsum, and the mass of CaSO4 in the liquid solution.
- Calculate the percentage recovery of CaSO4—that is, the percentage of the total CaSO4(precipitated plus dissolved) leaving the crystallizer recovered as solid anhydrous CaSO4.
- List five potential negative consequences of discharging H2SO4 into the river passing the plant.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
ELEM.PRIN.OF CHEM.PROCESS-ACCESS
Additional Engineering Textbook Solutions
Database Concepts (8th Edition)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Starting Out with C++ from Control Structures to Objects (9th Edition)
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Starting Out with Programming Logic and Design (5th Edition) (What's New in Computer Science)
- Can you help me? I can't seem to understand the handwriting for the five problems, and I want to be able to solve them and practice. If you'd like to give me steps, please do so to make it easier understand.arrow_forwardThe number of 2sp3 hybrid orbitals in the moleculeis A. 12; B. 8; C. 3; D. 11; E. None of the other answers is correct.arrow_forwardNonearrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning



