
Concept explainers
(a)
Interpretation: Nature of below alkyl radical along with names and most stable radical should be described by orbital description.

Concept introduction: The carbon radical linked to one alkyl/carbon while other two
The carbon radical linked to two alkyl/carbon atoms and one
The various kinds of alkyl radicals are indicated below:
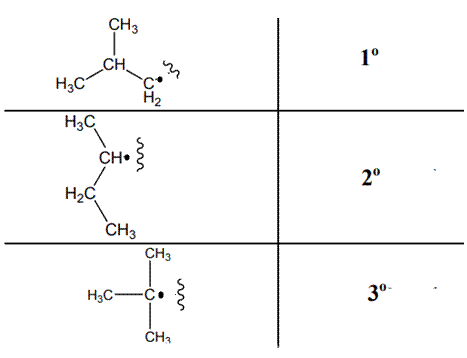
The tertiary radicals are most stable followed by secondary and least stable is primary methyl radical. This is because there is maximum stabilization through delocalization via hyperconjugation. The phenomenon of hyperconjugation refers to donation of
(b)
Interpretation: Nature of below alkyl radical along with names and most stable radical should be described by orbital description.
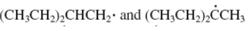
Concept introduction: The carbon radical linked to one alkyl/carbon while other two
The carbon radical linked to two alkyl/carbon atoms and one
The various kinds of alkyl radicals are indicated below:
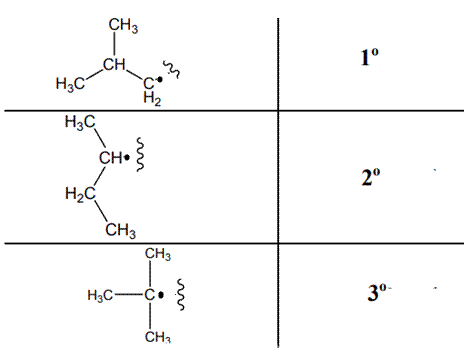
The tertiary radicals are most stable followed by secondary and least stable is primary methyl radical. This is because there is maximum stabilization through delocalization via hyperconjugation. The phenomenon of hyperconjugation refers to donation of
(c)
Interpretation: Nature of below alkyl radical along with names and most stable radical should be described by orbital description.
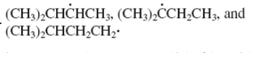
Concept introduction: The carbon radical linked to one alkyl/carbon while other two
The carbon radical linked to two alkyl/carbon atoms and one
The various kinds of alkyl radicals are indicated below:
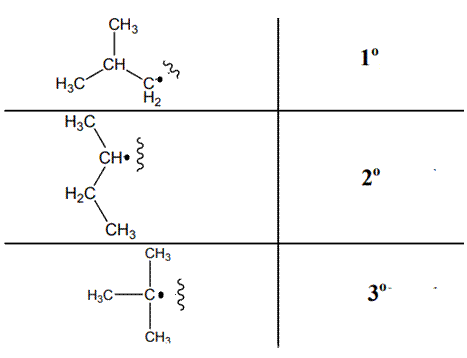
The tertiary radicals are most stable followed by secondary and least stable is primary methyl radical. This is because there is maximum stabilization through delocalization via hyper conjugation. The phenomenon of hyperconjugation refers to donation of
Trending nowThis is a popular solution!

Chapter 3 Solutions
EBK ORGANIC CHEMISTRY
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.200 M HClarrow_forwardCalculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forwardCan I please get help with this?arrow_forwardCan I please get help with this?arrow_forward
- Use the Henderson-Hasselbalch equation to calculate pH of a buffer containing 0.050M benzoic acidand 0.150M sodium benzoate. The Ka of benzoic acid is 6.5 x 10-5arrow_forwardA. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forward
- Can I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

