
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 29, Problem 29.67P
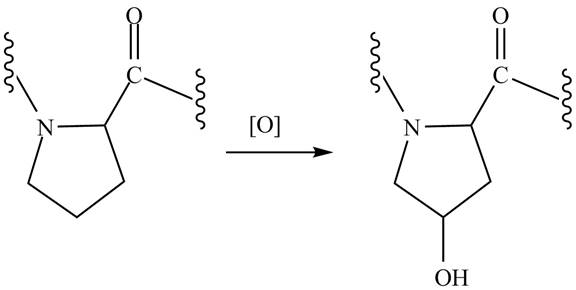
After the peptide chain of collagen has been formed, many of the proline residues are hydroxylated on one of the ring carbon atoms. Why is this process important for the triple helix of collagen?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can you show me or determine the longest carbon chain, which is octane? Potentially highlight it in different sections to show me, plz, or individually?
PLEASE ANSWER ALL PARTS!!
d) Determine the formal charge on the nitrogen atom in each of the structures.
NH3
NH2
N
C
бобкат
: N
N
H
H
Н
H2N-OH
A
B
C
D
E
F
G
Chapter 29 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 29 - Prob. 29.1PCh. 29 - Problem 29.2
What form exists at the isoelectric...Ch. 29 - Problem 29.3
Explain why the of the group of an...Ch. 29 - Prob. 29.4PCh. 29 - Problem 29.5
What -halo carbonyl compound is...Ch. 29 - Problem 29.6
The enolate derived from diethyl...Ch. 29 - Problem 29.7
What amino acid is formed when is...Ch. 29 - Problem 29.8
What aldehyde is needed to synthesize...Ch. 29 - Problem 29.9
Draw the products of each...Ch. 29 - Prob. 29.10P
Ch. 29 - Prob. 29.11PCh. 29 - Prob. 29.12PCh. 29 - Problem 29.13
What alkene is needed to synthesize...Ch. 29 - Problem 29.14
Draw the structure of each peptide....Ch. 29 - Problem 29.15
Name each peptide using both the...Ch. 29 - Prob. 29.16PCh. 29 - Prob. 29.17PCh. 29 - Problem 29.18
Glutathione, a powerful antioxidant...Ch. 29 - Problem 29.19
Draw the structure of the...Ch. 29 - Problem 29.20
Give the amino acid sequence of an...Ch. 29 - a What products are formed when each peptide is...Ch. 29 - Prob. 29.22PCh. 29 - Devise a synthesis of each peptide from amino acid...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.25PCh. 29 - Consider two molecules of a tetrapeptide composed...Ch. 29 - What types of stabilizing interactions exist...Ch. 29 - Prob. 29.28PCh. 29 - Draw the product formed when the following amino...Ch. 29 - With reference to the following peptide: a...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.32PCh. 29 - Histidine is classified as a basic amino acid...Ch. 29 - Tryptophan is not classified as a basic amino acid...Ch. 29 - What is the structure of each amino acid at its...Ch. 29 - What is the predominant form of each of the...Ch. 29 - 29.37 What is the predominant form of each of the...Ch. 29 - a. Draw the structure of the tripeptide A–A–A, and...Ch. 29 - 29.39 Draw the organic products formed in each...Ch. 29 - 29.40 What alkyl halide is needed to synthesize...Ch. 29 - 29.41 Devise a synthesis of threonine from diethyl...Ch. 29 - 29.42 Devise a synthesis of each amino acid from...Ch. 29 - Prob. 29.43PCh. 29 - Prob. 29.44PCh. 29 - Prob. 29.45PCh. 29 - Prob. 29.46PCh. 29 - Prob. 29.47PCh. 29 - 29.48 Brucine is a poisonous alkaloid obtained...Ch. 29 - Prob. 29.49PCh. 29 - Prob. 29.50PCh. 29 - Draw the structure for each peptide: (a) Phe–Ala;...Ch. 29 - 29.52 For the tetrapeptide Asp–Arg–Val–Tyr:
a....Ch. 29 - Prob. 29.53PCh. 29 - Prob. 29.54PCh. 29 - 29.55 Draw the amino acids and peptide fragments...Ch. 29 - Prob. 29.56PCh. 29 - Prob. 29.57PCh. 29 - Prob. 29.58PCh. 29 - 29.59 An octapeptide contains the following amino...Ch. 29 - 29.60 Draw the organic products formed in each...Ch. 29 - Draw all the steps in the synthesis of each...Ch. 29 - 29.62 Write out the steps for the synthesis of...Ch. 29 - 29.63 Besides the Boc and Fmoc protecting groups...Ch. 29 - 29.64 Another method to form a peptide bond...Ch. 29 - 29.65 Draw the mechanism for the reaction that...Ch. 29 - 29.66 Which of the following amino acids are...Ch. 29 - 29.67 After the peptide chain of collagen has been...Ch. 29 - Prob. 29.68PCh. 29 - Prob. 29.69PCh. 29 - 29.70 The anti-obesity drug orlistat works by...Ch. 29 - Prob. 29.71P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Lewis Structure, Hybridization & Molecular Geometry a) Draw the Lewis Structure of the molecules; Label the hybridization of each carbon atom; Predict the approximate molecular geometry around each carbon atom. CH3CHO CH3CN b) Draw the Lewis Structure of Nitromethane; Predict the approximate molecular geometry around the nitrogen atom. CH3NO2 c) Draw the Lewis Structure; Label the hybridization of the boron atom; Predict the approximate molecular geometry. BF3 BF4arrow_forwarda. The structure of the bicarbonate (hydrogen carbonate) ion, HCO3-, HCO3 " is best described as a hybrid of several contributing resonance forms, two of which are shown here. HO :0: HO + :Ö: Bicarbonate is crucial for the control of body pH (for example, blood pH 7.4). A more self-indulgent use is in baking soda, where it serves as a source of CO2 CO2 gas, which gives bread and pastry their fluffy constituency. (i) Draw at least one additional resonance form. = (ii) Using curved "electron-pushing" arrows, show how these Lewis structures may be interconverted by movement of electron pairs. (iii) Determine which form or forms will be the major contributor(s) to the real structure of bicarbonate, explaining your answer on the basis of the criteria in Section 1-5.arrow_forwardCalibri 11 + BIL NAME: Jaylena M A student is investigating the ctect of volume on pressure during a lab activity. The student uses the following volumes (mL). 12, 13, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 26, 28, 30, 33, 34, 35, 38, 40, 42, 44. 46, and 50. As the volume changed they measured the following pressures (atm) 11.0, 10.5, 10.0, 9.2. 8.5, 78, 75, 7.0, 6.8, 6.5, 6.0, 5.9, 5.5, 5.0, 4.8, 4.5, 4.2, 3.9, 3.8, 3.5, 3.3, 3.2, 3.0, 2.9. What is the independent variable? Volume Imla What is the dependent variable? Pressure Jatm Use the data and make a PROPER data table. Volume 1mL) Pressure latm 110arrow_forward
- Draw all resonance forms of the molecules. Include curved arrow notation. Label major resonance contributor.arrow_forward: Resonance Forms a) Draw all resonance forms of the molecules. Include curved arrow notation. Label major resonance contributor. SO₂ NO3arrow_forward1d. Use Le Chatelier's principle to describe the effect of the following changes on the position of the Haber-Bosch equilibrium: N2(g) + 3H2(g)= 2NH3(9) AH = -92kJ Choose one of the following answers: shift to reactant side, shift to product side or no change and draw the resulting graph. I. Increase the [N2(g)] Effect: H₂ N₂ NH3 II. Decrease the volume of the container. Effect: H₂ N₂2 NH3arrow_forward
- f) The unusual molecule [2.2.2] propellane is pictured. 1) Given the bond length and bond angles in the image, what hybridization scheme best describes the carbons marked by the askerisks? 2) What types of orbitals are used in the bond between the two carbons marked by the askerisks? 3) How does this bond compare to an ordinary carbon-carbon bond (which is usually 1.54 Å long)? CH2 1.60Å H₂C * H₂C CH2 C H2C * C Of H₂ 120°arrow_forwarde) Determine the hybridization and geometry around the indicated carbon atoms. H3C CH3 B HC CH2 A C C C CH3arrow_forwardDon't used Ai solution and hand raitingarrow_forward
- Don't used Ai solutionarrow_forwardDon't used Ai solution and hand raitingarrow_forward75.0 grams of an unknown metal was heated to 95.0°C, it was then placed into 150.0 grams of water at23.1°C, when the metal and water reached thermal equilibrium, the temperature was 27.8°C. Calculatethe specific heat of the metal. (Assume that the specific heat of water is 4.18 J/g °C)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY