
(a)
Interpretation:
The given reaction has to be identified as electro cyclic reaction, a cycloaddition reaction or a sigma tropic reaction.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or broken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In an electrocyclic reaction “one new sigma- bond is formed or broken.”
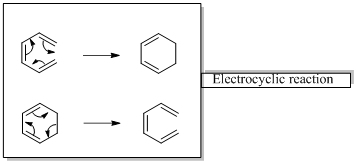
In a cycloaddition reaction “ two new sigma-bonds are formed or broken”
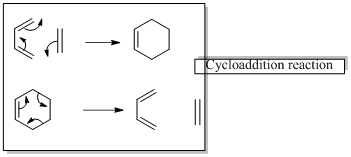
In a sigmatropic rearrangement reaction “ one new sigma-bond is formed as another breaks.”
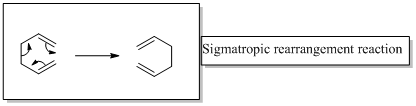
(b)
Interpretation: The given reaction has to be identified as electro cyclic reaction, a cycloaddition reaction or a sigma tropic reaction.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or broken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In an electrocyclic reaction “one new sigma- bond is formed or broken.”

In a cycloaddition reaction “ two new sigma-bonds are formed or broken”

In a sigmatropic rearrangement reaction “ one new sigma-bond is formed as another breaks.”

(c)
Interpretation: The given reaction has to be identified as electro cyclic reaction, a cycloaddition reaction or a sigma tropic reaction.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or broken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In an electrocyclic reaction “one new sigma- bond is formed or broken.”

In a cycloaddition reaction “ two new sigma-bonds are formed or broken”

In a sigmatropic rearrangement reaction “ one new sigma-bond is formed as another breaks.”

(d)
Interpretation: The given reaction has to be identified as electro cyclic reaction, a cycloaddition reaction or a sigma tropic reaction.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or broken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In an electrocyclic reaction “one new sigma- bond is formed or broken.”

In a cycloaddition reaction “ two new sigma-bonds are formed or broken”

In a sigmatropic rearrangement reaction “ one new sigma-bond is formed as another breaks.”

Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forward
- Add conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward3. Provide all the steps and reagents for this synthesis. OHarrow_forwardSteps and explanationarrow_forward
- Steps and explanations please.arrow_forwardSteps on how to solve. Thank you!arrow_forward3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward
