
(a)
Interpretation:
It should be identified that will a thermal 1, 3-migrations of carbon occur with whether retention or inversion of configuration.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
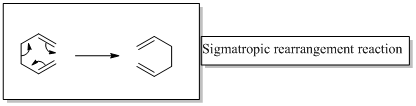
Sigmatropic rearrangement reactions are named with digits. For example a [1, 3] sigmatropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3.
Migration of carbon and hydrogen will occur in a sigmatropic rearrangement reaction.
When hydrogen migrates in a sigmatropic rearrangement, the s orbital of the hydrogen is partially bonded to both the migration origin and the migration terminus in the transition state.
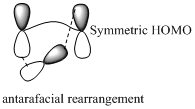
Migration of hydrogen in suprafacial and antarafacial rearrangement can be represented as follows,


Migration of carbon occurs through two ways because it has a two lobed p orbital. Carbon can simultaneously interact with the migration origin and the migration terminus using one lobe of its p orbital.
Migration of carbon in suprafacial and antarafacial rearrangement can be represented as follows,
Carbon migrating with one lobe of its p orbital interacting


Carbon migrating with both lobe of its p orbital interacting

Inversion of configuration is the inversion of a chiral center in a molecule in a
Retention of configuration is the preservation of integrity of the spatial arrangement of bonds to a chiral center during a chemical reaction.
(b)
Interpretation:
It should be identified that will a thermal 1, 5-migrations of carbon occur with whether retention or inversion of configuration.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”

Sigmatropic rearrangement reactions are named with digits. For example a [1, 3] sigmatropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3.
Migration of carbon and hydrogen will occur in a sigmatropic rearrangement reaction.
When hydrogen migrates in a sigmatropic rearrangement, the s orbital of the hydrogen is partially bonded to both the migration origin and the migration terminus in the transition state.
Migration of hydrogen in suprafacial and antarafacial rearrangement can be represented as follows,


Migration of carbon occurs through two ways because it has a two lobed p orbital. Carbon can simultaneously interact with the migration origin and the migration terminus using one lobe of its p orbital.
Migration of carbon in suprafacial and antarafacial rearrangement can be represented as follows,
Carbon migrating with one lobe of its p orbital interacting


Carbon migrating with both lobe of its p orbital interacting


Inversion of configuration is the inversion of a chiral center in a molecule in a chemical reaction.
Retention of configuration is the preservation of integrity of the spatial arrangement of bonds to a chiral center during a chemical reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

