
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
8th Edition
ISBN: 9780134581064
Author: Bruice, Paula Yurkanis
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 28, Problem 27P
Interpretation Introduction
Interpretation:
The conversion of. reactant to product by two steps should be shown.
Concept introduction:
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
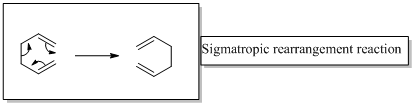
Sigmatropic rearrangement reactions are designated with digits. For example a [1, 3] sigmatropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3
[3,3] sigmatropic rearrangement of a 1,3-diene known as Cope rearrangement reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
how to get limiting reactant and %
yield based off this data
Compound
Mass 6) Volume(mL
Ben zaphone-5008
ne
Acetic Acid
1. Sam L
2-propanot
8.00
Benzopin-
a col
030445
Benzopin
a Colone 0.06743
Results
Compound
Melting Point (°c)
Benzopin
acol
172°c - 175.8 °c
Benzoping
to lone
1797-180.9
Assign ALL signals for the proton and carbon NMR spectra on the following pages.
7.5
1.93
2.05
C
B
A
4
3
5
The Joh.
9
7
8
1
2
7.5
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
1.5
1.0 ppm
9
7
8
0.86
OH 10
4
3
5
1
2
7.5
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
1.5
1.0
ppm
9
7
8
CI
4
3
5
1
2
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
2.21
4.00
1.5
2.00
2.07
1.0
ppm
2.76
Chapter 28 Solutions
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
Ch. 28.1 - Prob. 1PCh. 28.2 - Prob. 2PCh. 28.2 - Prob. 3PCh. 28.2 - Give a molecular orbital description for each of...Ch. 28.3 - Prob. 5PCh. 28.3 - Prob. 6PCh. 28.3 - Prob. 7PCh. 28.3 - Prob. 8PCh. 28.4 - Prob. 10PCh. 28.4 - Prob. 11P
Ch. 28.5 - Prob. 12PCh. 28.5 - a. Draw the product of the following reaction: b....Ch. 28.5 - Prob. 14PCh. 28.5 - Prob. 15PCh. 28.5 - Prob. 17PCh. 28.5 - Prob. 18PCh. 28.6 - Prob. 19PCh. 28.6 - Explain why the hydrogen and the methyl...Ch. 28.6 - Chorismate mutase is an enzyme that promotes a...Ch. 28.7 - Convince yourself that the TE-AC method for...Ch. 28 - Draw the product of each of the following...Ch. 28 - Draw the product of each of the following...Ch. 28 - Prob. 25PCh. 28 - Show how norbornance can be prepared from...Ch. 28 - Prob. 27PCh. 28 - Prob. 28PCh. 28 - Draw the product of each of the following...Ch. 28 - Prob. 30PCh. 28 - Prob. 31PCh. 28 - Prob. 32PCh. 28 - Prob. 33PCh. 28 - When the following compound is heated, a product...Ch. 28 - Prob. 35PCh. 28 - Propose a mechanism for the following reaction:Ch. 28 - Prob. 37PCh. 28 - Prob. 38PCh. 28 - Prob. 39PCh. 28 - Prob. 40PCh. 28 - If isomer A is heated to about 100 C, a mixture of...Ch. 28 - Propose a mechanism for the following reaction:Ch. 28 - Prob. 43PCh. 28 - A student found that heating any one of the...Ch. 28 - Prob. 45PCh. 28 - Prob. 46PCh. 28 - Prob. 47P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Assign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
- Order the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
