
Concept explainers
(a)
Interpretation: The given compound is to be classified as identical to
Concept introduction: The stereochemistry of the compound is determined by prioritizing the groups attached to its stereogenic center. The groups are prioritized on the basis of
Answer to Problem 28.39P
The given compound is classified as identical to
Explanation of Solution
The structure of compound
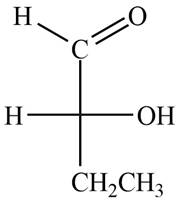
Figure 1
In the compound
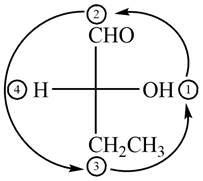
Figure 2
The circle rotates in the anticlockwise direction and its configuration will be
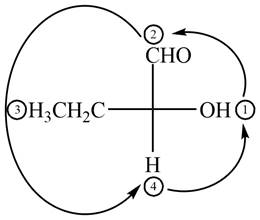
Figure 3
Thus, the configuration will reverse. Therefore, the compound
The given compound is,
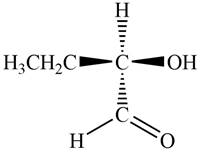
Figure 4
In the given compound,
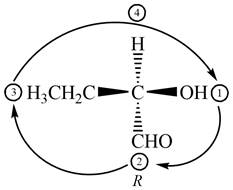
Figure 5
The circle rotates in the clockwise direction. Thus, the given compound is labeled as
Hence, the given compound is classified as identical to
The given compound is classified as identical to
(b)
Interpretation: The given compound is to be classified as identical to
Concept introduction: The stereochemistry of the compound is determined by prioritizing the groups attached to its stereogenic center. The groups are prioritized on the basis of atomic number of their atoms. The group that contain atom with higher atomic number is given higher priority. Complete the circle in decreasing order of priority from
Answer to Problem 28.39P
The given compound is classified as identical to
Explanation of Solution
The compound
The given compound is,
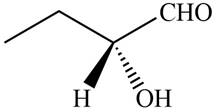
Figure 6
In the given compound,
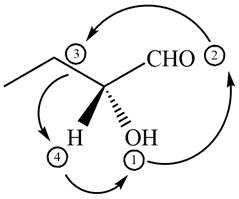
Figure 7
The circle rotates in the anticlockwise direction hence its configuration will be
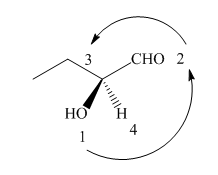
Figure 8
Thus, the configuration will reverse. Therefore, the compound is labeled as
Hence, the given compound is classified as identical to
The given compound is classified as identical to
(c)
Interpretation: The given compound is to be classified as identical to
Concept introduction: The stereochemistry of the compound is determined by prioritizing the groups attached to its stereogenic center. The groups are prioritized on the basis of atomic number of their atoms. The group that contain atom with higher atomic number is given higher priority. Complete the circle in decreasing order of priority from
Answer to Problem 28.39P
The given compound is classified as enantiomer to
Explanation of Solution
The compound
The given compound is,
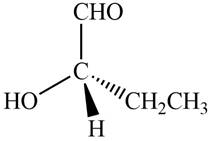
Figure 9
In the given compound,
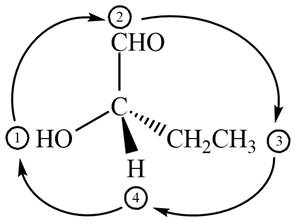
Figure 10
The circle rotates in the clockwise direction hence its configuration will be
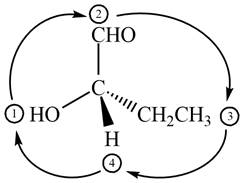
Figure 11
Thus, the configuration will reverse. Therefore, the compound is labeled as
The given compound and
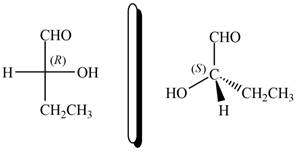
Figure 12
Hence, the given compound is classified as enantiomer to
The given compound is classified as enantiomer to
Want to see more full solutions like this?
Chapter 28 Solutions
ORGANIC CHEMISTRY
- Look at the image attached pleaarrow_forwardComplete the mechanismarrow_forwardV Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forward
- Complete the mechanismarrow_forwardComplete the mechanismarrow_forward8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





