
Thermodynamics: An Engineering Approach
8th Edition
ISBN: 9780073398174
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.8, Problem 107P
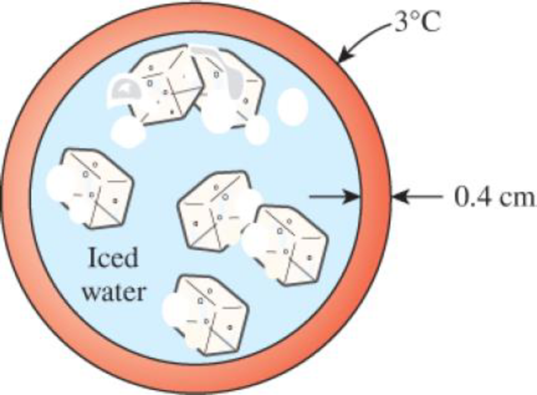
A hollow spherical iron container whose outer diameter is 40 cm and thickness is 0.4 cm is filled with iced water at 0°C. If the outer surface temperature is 3°C, determine the approximate rate of heat loss from the sphere, and the rate at which ice melts in the container.
FIGURE P2–107
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
32 mm
32 mm
b'
c'
C
32 mm
32 mm
b
PROBLEM 6.41
a
The extruded beam shown has a uniform wall thickness of 3 mm. Knowing that
the vertical shear in the beam is 9 kN, determine the shearing stress at each of the
five points indicated.
In a structural reliability problem, the resistance (capacity) R and load effect (demand) S random variables
associated with a failure mode of the structure of interest are normally distributed and statistically
independent with the following probability distribution parameters (or statistics) in consistent units:
MR = 12, σR = 3
μs = 5, σs = 2
(a) Determine the exact probability of failure pF ·
The resistance R and load effect S for a given failure mode are statistically independent random variables
with marginal PDF's
1
fR (r) =
0≤r≤100
100'
fs(s)=0.05e-0.05s
(a) Determine the probability of failure by computing the probability content of the failure domain defined
as {r
Chapter 2 Solutions
Thermodynamics: An Engineering Approach
Ch. 2.8 - What is total energy? Identify the different forms...Ch. 2.8 - List the forms of energy that contribute to the...Ch. 2.8 - How are heat, internal energy, and thermal energy...Ch. 2.8 - What is mechanical energy? How does it differ from...Ch. 2.8 - Natural gas, which is mostly methane CH4, is a...Ch. 2.8 - Portable electric heaters are commonly used to...Ch. 2.8 - Prob. 7PCh. 2.8 - Prob. 8PCh. 2.8 - 2–9E Calculate the total potential energy, in Btu,...Ch. 2.8 - Prob. 10P
Ch. 2.8 - Prob. 11PCh. 2.8 - At a certain location, wind is blowing steadily at...Ch. 2.8 - A water jet that leaves a nozzle at 60 m/s at a...Ch. 2.8 - Prob. 14PCh. 2.8 - Prob. 15PCh. 2.8 - Consider a river flowing toward a lake at an...Ch. 2.8 - When is the energy crossing the boundaries of a...Ch. 2.8 - Consider an automobile traveling at a constant...Ch. 2.8 - A gas in a pistoncylinder device is compressed,...Ch. 2.8 - A room is heated by an iron that is left plugged...Ch. 2.8 - A room is heated as a result of solar radiation...Ch. 2.8 - Prob. 23PCh. 2.8 - A small electrical motor produces 5 W of...Ch. 2.8 - Prob. 25PCh. 2.8 - 2–26C Lifting a weight to a height of 20 m takes...Ch. 2.8 - Prob. 27PCh. 2.8 - Prob. 28PCh. 2.8 - Prob. 29PCh. 2.8 - Prob. 30PCh. 2.8 - Prob. 31PCh. 2.8 - Prob. 32PCh. 2.8 - Prob. 33PCh. 2.8 - A ski lift has a one-way length of 1 km and a...Ch. 2.8 - The engine of a 1500-kg automobile has a power...Ch. 2.8 - Prob. 36PCh. 2.8 - What are the different mechanisms for transferring...Ch. 2.8 - On a hot summer day, a student turns his fan on...Ch. 2.8 - Prob. 39PCh. 2.8 - A vertical pistoncylinder device contains water...Ch. 2.8 - At winter design conditions, a house is projected...Ch. 2.8 - A water pump increases the water pressure from 15...Ch. 2.8 - Prob. 43PCh. 2.8 - Prob. 44PCh. 2.8 - A university campus has 200 classrooms and 400...Ch. 2.8 - Prob. 46PCh. 2.8 - Consider a room that is initially at the outdoor...Ch. 2.8 - Prob. 48PCh. 2.8 - 2-49 The 60-W fan of a central heating system is...Ch. 2.8 - Prob. 50PCh. 2.8 - An escalator in a shopping center is designed to...Ch. 2.8 - Prob. 52PCh. 2.8 - How is the combined pumpmotor efficiency of a pump...Ch. 2.8 - Prob. 54PCh. 2.8 - Can the combined turbinegenerator efficiency be...Ch. 2.8 - Consider a 2.4-kW hooded electric open burner in...Ch. 2.8 - Prob. 57PCh. 2.8 - Prob. 58PCh. 2.8 - Prob. 59PCh. 2.8 - A geothermal pump is used to pump brine whose...Ch. 2.8 - Prob. 62PCh. 2.8 - Prob. 63PCh. 2.8 - The water in a large lake is to be used to...Ch. 2.8 - A 7-hp (shaft) pump is used to raise water to an...Ch. 2.8 - At a certain location, wind is blowing steadily at...Ch. 2.8 - Reconsider Prob. 265. Using appropriate software,...Ch. 2.8 - Water is pumped from a lake to a storage tank 15 m...Ch. 2.8 - Prob. 69PCh. 2.8 - A hydraulic turbine has 85 m of elevation...Ch. 2.8 - Prob. 71PCh. 2.8 - Water is pumped from a lower reservoir to a higher...Ch. 2.8 - Prob. 73PCh. 2.8 - An oil pump is drawing 44 kW of electric power...Ch. 2.8 - How does energy conversion affect the environment?...Ch. 2.8 - What is acid rain? Why is it called a rain? How do...Ch. 2.8 - Why is carbon monoxide a dangerous air pollutant?...Ch. 2.8 - What is the greenhouse effect? How does the excess...Ch. 2.8 - What is smog? What does it consist of? How does...Ch. 2.8 - Prob. 80PCh. 2.8 - Consider a household that uses 14,000 kWh of...Ch. 2.8 - When a hydrocarbon fuel is burned, almost all of...Ch. 2.8 - Prob. 83PCh. 2.8 - A typical car driven 20,000 km a year emits to the...Ch. 2.8 - What are the mechanisms of heat transfer?Ch. 2.8 - Which is a better heat conductor, diamond or...Ch. 2.8 - How does forced convection differ from natural...Ch. 2.8 - What is a blackbody? How do real bodies differ...Ch. 2.8 - Define emissivity and absorptivity. What is...Ch. 2.8 - Does any of the energy of the sun reach the earth...Ch. 2.8 - The inner and outer surfaces of a 5-m 6-m brick...Ch. 2.8 - The inner and outer surfaces of a 0.5-cm-thick 2-m...Ch. 2.8 - Reconsider Prob. 292. Using appropriate software,...Ch. 2.8 - Prob. 94PCh. 2.8 - Prob. 95PCh. 2.8 - Prob. 96PCh. 2.8 - Prob. 97PCh. 2.8 - For heat transfer purposes, a standing man can be...Ch. 2.8 - Prob. 99PCh. 2.8 - Prob. 100PCh. 2.8 - A 1000-W iron is left on the ironing board with...Ch. 2.8 - A 7-cm-external-diameter, 18-m-long hot-water pipe...Ch. 2.8 - A thin metal plate is insulated on the back and...Ch. 2.8 - Reconsider Prob. 2103. Using appropriate software,...Ch. 2.8 - The outer surface of a spacecraft in space has an...Ch. 2.8 - Prob. 106PCh. 2.8 - A hollow spherical iron container whose outer...Ch. 2.8 - Consider a vertical elevator whose cabin has a...Ch. 2.8 - Consider a homeowner who is replacing his...Ch. 2.8 - Prob. 110RPCh. 2.8 - Prob. 111RPCh. 2.8 - Prob. 112RPCh. 2.8 - 2–113 The U.S. Department of Energy estimates that...Ch. 2.8 - Prob. 114RPCh. 2.8 - Prob. 115RPCh. 2.8 - Prob. 116RPCh. 2.8 - Prob. 117RPCh. 2.8 - Consider a TV set that consumes 120 W of electric...Ch. 2.8 - Water is pumped from a 200-ft-deep well into a...Ch. 2.8 - Prob. 120RPCh. 2.8 - Prob. 121RPCh. 2.8 - In a hydroelectric power plant, 65 m3/s of water...Ch. 2.8 - The demand for electric power is usually much...Ch. 2.8 - The pump of a water distribution system is powered...Ch. 2.8 - On a hot summer day, the air in a well-sealed room...Ch. 2.8 - Prob. 126FEPCh. 2.8 - A 2-kW electric resistance heater in a room is...Ch. 2.8 - A 900-kg car cruising at a constant speed of 60...Ch. 2.8 - Prob. 129FEPCh. 2.8 - Prob. 130FEPCh. 2.8 - Prob. 131FEPCh. 2.8 - A 2-kW pump is used to pump kerosene ( = 0.820...Ch. 2.8 - Prob. 133FEPCh. 2.8 - Prob. 134FEPCh. 2.8 - Prob. 135FEPCh. 2.8 - Prob. 136FEPCh. 2.8 - Prob. 137FEPCh. 2.8 - Heat is transferred steadily through a...Ch. 2.8 - The roof of an electrically heated house is 7 m...
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
Using your text editor, enter (that is, type in) the C++ program shown in Display 1.8. Be certain to type the f...
Problem Solving with C++ (10th Edition)
A nozzle at A discharges water with an initial velocity of 36 ft/s at an angle with the horizontal. Determine ...
Vector Mechanics For Engineers
The solid steel shaft AC has a diameter of 25 mm and is supported by smooth bearings at D and E. It is coupled ...
Mechanics of Materials (10th Edition)
Write a summary list of the problem-solving steps identified in the chapter, using your own words.
BASIC BIOMECHANICS
In Exercises 1 through 22, determine the output displayed in the text box or list box by the lines of code.
Introduction To Programming Using Visual Basic (11th Edition)
What types of coolant are used in vehicles?
Automotive Technology: Principles, Diagnosis, And Service (6th Edition) (halderman Automotive Series)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Please solve this problem as soon as possible My ID# 016948724arrow_forwardThe gears shown in the figure have a diametral pitch of 2 teeth per inch and a 20° pressure angle. The pinion rotates at 1800 rev/min clockwise and transmits 200 hp through the idler pair to gear 5 on shaft c. What forces do gears 3 and 4 transmit to the idler shaft? TS I y 18T 32T This a 12 x 18T C 48T 5arrow_forwardQuestion 1. Draw 3 teeth for the following pinion and gear respectively. The teeth should be drawn near the pressure line so that the teeth from the pinion should mesh those of the gear. Drawing scale (1:1). Either a precise hand drawing or CAD drawing is acceptable. Draw all the trajectories of the involute lines and the circles. Specification: 18tooth pinion and 30tooth gear. Diameter pitch=P=6 teeth /inch. Pressure angle:20°, 1/P for addendum (a) and 1.25/P for dedendum (b). For fillet, c=b-a.arrow_forward
- 5. The figure shows a gear train. There is no friction at the bearings except for the gear tooth forces. The material of the milled gears is steel having a Brinell hardness of 170. The input shaft speed (n2) is 800 rpm. The face width and the contact angle for all gears are 1 in and 20° respectively. In this gear set, the endurance limit (Se) is 15 kpsi and nd (design factor) is 2. (a) Find the revolution speed of gear 5. (b) Determine whether each gear satisfies the design factor of 2.0 for bending fatigue. (c) Determine whether each gear satisfies the design factor of 2.0 for surface fatigue (contact stress). (d) According to the computation results of the questions (b) and (c), explain the possible failure mechanisms for each gear. N4=28 800rpm N₁=43 N5=34 N₂=14 P(diameteral pitch)=8 for all gears Coupled to 2.5hp motorarrow_forward1. The rotating steel shaft is simply supported by bearings at points of B and C, and is driven by a spur gear at D, which has a 6-in pitch diameter. The force F from the drive gear acts at a pressure angle of 20°. The shaft transmits a torque to point A of TA =3000 lbĘ in. The shaft is machined from steel with Sy=60kpsi and Sut=80 kpsi. (1) Draw a shear force diagram and a bending moment diagram by F. According to your analysis, where is the point of interest to evaluate the safety factor among A, B, C, and D? Describe the reason. (Hint: To find F, the torque Tд is generated by the tangential force of F (i.e. Ftangential-Fcos20°) When n=2.5, K=1.8, and K₁ =1.3, determine the diameter of the shaft based on (2) static analysis using DE theory (note that fatigue stress concentration factors need to be used for this question because the loading condition is fatigue) and (3) a fatigue analysis using modified Goodman. Note) A standard diameter is not required for the questions. 10 in Darrow_forward3 N2=28 P(diametral pitch)=8 for all gears Coupled to 25 hp motor N3=34 Full depth spur gears with pressure angle=20° N₂=2000 rpm (1) Compute the circular pitch, the center-to-center distance, and base circle radii. (2) Draw the free body diagram of gear 3 and show all the forces and the torque. (3) In mounting gears, the center-to-center distance was reduced by 0.1 inch. Calculate the new values of center-to-center distance, pressure angle, base circle radii, and pitch circle diameters. (4)What is the new tangential and radial forces for gear 3? (5) Under the new center to center distance, is the contact ratio (mc) increasing or decreasing?arrow_forward
- 2. A flat belt drive consists of two 4-ft diameter cast-iron pulleys spaced 16 ft apart. A power of 60 hp is transmitted by a pulley whose speed is 380 rev/min. Use a service factor (Ks) pf 1.1 and a design factor 1.0. The width of the polyamide A-3 belt is 6 in. Use CD=1. Answer the following questions. (1) What is the total length of the belt according to the given geometry? (2) Find the centrifugal force (Fc) applied to the belt. (3) What is the transmitted torque through the pulley system given 60hp? (4) Using the allowable tension, find the force (F₁) on the tight side. What is the tension at the loose side (F2) and the initial tension (F.)? (5) Using the forces, estimate the developed friction coefficient (f) (6) Based on the forces and the given rotational speed, rate the pulley set. In other words, what is the horse power that can be transmitted by the pulley system? (7) To reduce the applied tension on the tight side, the friction coefficient is increased to 0.75. Find out the…arrow_forwardThe tooth numbers for the gear train illustrated are N₂ = 24, N3 = 18, №4 = 30, №6 = 36, and N₁ = 54. Gear 7 is fixed. If shaft b is turned through 5 revolutions, how many turns will shaft a make? a 5 [6] barrow_forwardCE-112 please solve this problem step by step and give me the correct answerarrow_forward
- CE-112 please solve this problem step by step and give me the correct answerarrow_forwardCE-112 solve this problem step by step and give me the correct answer pleasearrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license