
Organic Chemistry, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134074580
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27.5, Problem 8P
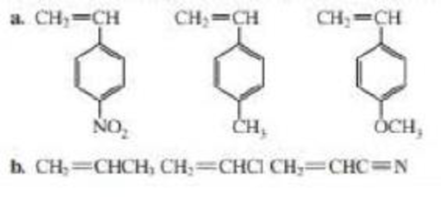
Rank the following groups of monomers from most able to least able to undergo anionic
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Curved arrows are used to illustrate the flow of electrons. Using
the provided starting and product structures, draw the curved
electron-pushing arrows for the following reaction or
mechanistic step(s).
Be sure to account for all bond-breaking and bond-making
steps.
Prob
10:
Select to Add Arrows
THE
Curved arrows are used to illustrate the flow of electrons using the provided starting and product structures draw the curved electron pushing arrows for the following reaction or mechanistic steps Ether(solvent)
This deals with synthetic organic chemistry. Please fill in the blanks appropriately.
Chapter 27 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
Ch. 27.3 - Prob. 1PCh. 27.3 - Prob. 2PCh. 27.3 - Prob. 3PCh. 27.3 - Prob. 4PCh. 27.3 - Prob. 5PCh. 27.3 - Prob. 6PCh. 27.4 - Prob. 7PCh. 27.5 - Rank the following groups of monomers from most...Ch. 27.5 - Why does methyl methacrylate not undergo cationic...Ch. 27.6 - Prob. 10P
Ch. 27.6 - Explain why, when propylene oxide undergoes...Ch. 27.6 - Which monomer and which type of initiator can you...Ch. 27.6 - Prob. 13PCh. 27.8 - Draw a short segment of gutta-percha.Ch. 27.8 - Prob. 15PCh. 27.11 - Prob. 16PCh. 27.11 - Write an equation that explains what happens if a...Ch. 27.11 - What happens to polyester slacks if aqueous NaOH...Ch. 27.11 - a. Propose a mechanism for the formation of the...Ch. 27.11 - Explain why, when a small amount of glycerol is...Ch. 27.12 - Propose a mechanism for the formation of melmac.Ch. 27.12 - Prob. 22PCh. 27.13 - Prob. 23PCh. 27 - Draw short segments of the polymers obtained from...Ch. 27 - Prob. 25PCh. 27 - Prob. 26PCh. 27 - Draw the structure of the monomer or monomers used...Ch. 27 - Prob. 28PCh. 27 - Draw short segments of the polymers obtained from...Ch. 27 - Quiana is a synthetic fabric that feels very much...Ch. 27 - Prob. 31PCh. 27 - Prob. 32PCh. 27 - Prob. 33PCh. 27 - Poly(vinyl alcohol) is a polymer used to make...Ch. 27 - Five different repeating units are found in the...Ch. 27 - Prob. 37PCh. 27 - A particularly strong and rigid polyester used for...Ch. 27 - Prob. 39PCh. 27 - Which Monomer gives a greater yield of polymer,...Ch. 27 - Prob. 41PCh. 27 - Prob. 42PCh. 27 - Why do vinyl raincoats become brittle as they get...Ch. 27 - The polymer shown below is synthesized by...Ch. 27 - Prob. 45PCh. 27 - How can head-to-head poly(vinyl bromide) be...Ch. 27 - Delrin (polyoxymethylene) is a tough...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the References to access important values if needed for this question. What is the IUPAC name of each of the the following? 0 CH3CHCNH₂ CH3 CH3CHCNHCH2CH3 CH3arrow_forwardYou have now performed a liquid-liquid extraction protocol in Experiment 4. In doing so, you manipulated and exploited the acid-base chemistry of one or more of the compounds in your mixture to facilitate their separation into different phases. The key to understanding how liquid- liquid extractions work is by knowing which layer a compound is in, and in what protonation state. The following liquid-liquid extraction is different from the one you performed in Experiment 4, but it uses the same type of logic. Your task is to show how to separate apart Compound A and Compound B. . Complete the following flowchart of a liquid-liquid extraction. Handwritten work is encouraged. • Draw by hand (neatly) only the appropriate organic compound(s) in the boxes. . Specify the reagent(s)/chemicals (name is fine) and concentration as required in Boxes 4 and 5. • Box 7a requires the solvent (name is fine). • Box 7b requires one inorganic compound. • You can neatly complete this assignment by hand and…arrow_forwardb) Elucidate compound D w) mt at 170 nd shows c-1 stretch at 550cm;' The compound has the ff electronic transitions: 0%o* and no a* 1H NMR Spectrum (CDCl3, 400 MHz) 3.5 3.0 2.5 2.0 1.5 1.0 0.5 ppm 13C{H} NMR Spectrum (CDCl3, 100 MHz) Solvent 80 70 60 50 40 30 20 10 0 ppm ppm ¹H-13C me-HSQC Spectrum ppm (CDCl3, 400 MHz) 5 ¹H-¹H COSY Spectrum (CDCl3, 400 MHz) 0.5 10 3.5 3.0 2.5 2.0 1.5 1.0 10 15 20 20 25 30 30 -35 -1.0 1.5 -2.0 -2.5 3.0 -3.5 0.5 ppm 3.5 3.0 2.5 2.0 1.5 1.0 0.5 ppmarrow_forward
- Part I. a) Elucidate the structure of compound A using the following information. • mass spectrum: m+ = 102, m/2=57 312=29 • IR spectrum: 1002.5 % TRANSMITTANCE Ngg 50 40 30 20 90 80 70 60 MICRONS 5 8 9 10 12 13 14 15 16 19 1740 cm M 10 0 4000 3600 3200 2800 2400 2000 1800 1600 13 • CNMR 'H -NMR Peak 8 ppm (H) Integration multiplicity a 1.5 (3H) triplet b 1.3 1.5 (3H) triplet C 2.3 1 (2H) quartet d 4.1 1 (2H) quartet & ppm (c) 10 15 28 60 177 (C=0) b) Elucidate the structure of compound B using the following information 13C/DEPT NMR 150.9 MHz IIL 1400 WAVENUMBERS (CM-1) DEPT-90 DEPT-135 85 80 75 70 65 60 55 50 45 40 35 30 25 20 ppm 1200 1000 800 600 400arrow_forward• Part II. a) Elucidate The structure of compound c w/ molecular formula C10 11202 and the following data below: • IR spectra % TRANSMITTANCE 1002.5 90 80 70 60 50 40 30 20 10 0 4000 3600 3200 2800 2400 2000 1800 1600 • Information from 'HAMR MICRONS 8 9 10 11 14 15 16 19 25 1400 WAVENUMBERS (CM-1) 1200 1000 800 600 400 peak 8 ppm Integration multiplicity a 2.1 1.5 (3H) Singlet b 3.6 1 (2H) singlet с 3.8 1.5 (3H) Singlet d 6.8 1(2H) doublet 7.1 1(2H) doublet Information from 13C-nmR Normal carbon 29ppm Dept 135 Dept -90 + NO peak NO peak 50 ppm 55 ppm + NO peak 114 ppm t 126 ppm No peak NO peak 130 ppm t + 159 ppm No peak NO peak 207 ppm по реак NO peakarrow_forwardCould you redraw these and also explain how to solve them for me pleasarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY