
Tutorials in Introductory Physics
1st Edition
ISBN: 9780130970695
Author: Peter S. Shaffer, Lillian C. McDermott
Publisher: Addison Wesley
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 27.2, Problem 1aTH
To determine
To Describe: Whether, an ideal gas process satisfies the given conditions or not.
Expert Solution & Answer
Explanation of Solution
Introduction:
The process with constant temperature and with some
Take an example of piston cylinder arrangement with an ideal gas. Gas is allowed to expand and contract. The heat is supplied or extract from the cylinder . This expands or contracts the piston slowly and produce work without change in temperature.
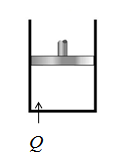
Conclusion:
Yes, in isothermal process the temperature is constant and heat is transferred.
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
A pendulum has a 0.4-m-long cord and is given a tangential velocity of 0.2 m/s toward the
vertical from a position 0 = 0.3 rad.
Part A
Determine the equation which describes the angular motion.
Express your answer in terms of the variable t. Express coefficients in radians to three significant figures.
ΜΕ ΑΣΦ
vec
(t)=0.3 cos (4.95t) + 0.101 sin (4.95t)
Submit Previous Answers Request Answer
× Incorrect; Try Again; 6 attempts remaining
Part A
■Review
The uniform 150-lb stone (rectangular block) is being turned over on its side by pulling the
vertical cable slowly upward until the stone begins to tip.
(Figure 1)
If it then falls freely (T = 0) from an essentially balanced at-rest position, determine the speed at which the corner A strikes the pad at B. The stone does not slip at its corner C as it falls. Suppose that height of the stone is
L = 1.2 ft.
Express your answer to three significant figures and include the appropriate units.
?
ft
VA 10.76
S
Submit Previous Answers Request Answer
× Incorrect; Try Again; 6 attempts remaining
Consider the circuit shown in the figure. The battery has emf ε = 69 volts and negligible internal resistance. The inductance is L = 0.4 H and the resistances are R 1 = 12 Ω and R 2 = 9.0 Ω. Initially the switch S is open and no currents flow. Then the switch is closed. After leaving the switch closed for a very long time, it is opened again. Just after it is opened, what is the current in R 1?
Chapter 27 Solutions
Tutorials in Introductory Physics
Ch. 27.1 - Prob. 1aTHCh. 27.1 - In this process, which of the quantities P, V, n,...Ch. 27.1 - Consider the following incorrect student...Ch. 27.1 - Explain why it is not possible to use the ideal...Ch. 27.1 - A long pin is used to hold the piston in place as...Ch. 27.1 - A long pin is used to hold the piston in place as...Ch. 27.1 - Prob. 2cTHCh. 27.2 - Prob. 1aTHCh. 27.2 - Prob. 1bTHCh. 27.2 - Prob. 1cTH
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A capacitor with a capacitance of C = 5.95×10−5 F is charged by connecting it to a 12.5 −V battery. The capacitor is then disconnected from the battery and connected across an inductor with an inductance of L = 1.55 H . At the time 2.35×10−2 s after the connection to the inductor is made, what is the current in the inductor? At that time, how much electrical energy is stored in the inductor?arrow_forwardCan someone help me with this question. Thanks.arrow_forwardCan someone help me with this question. Thanks.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill


College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning