
(a)
Interpretation: The starting materials that are needed to synthesize the given compound by a thermal
Concept introduction: A
Answer to Problem 27.40P
The starting materials that are needed to synthesize the given compound by a thermal
Explanation of Solution
The given product is shown below.
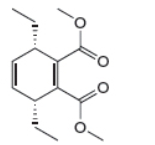
Figure 1
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
The reaction that shows the disconnection approach of the given product is shown below.
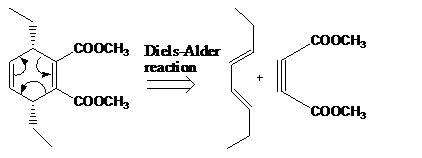
Figure 2
In the given product, the ring is opened due to the rearrangement of
The starting materials that are needed to synthesize the given compound by a thermal
(b)
Interpretation: The starting materials that are needed to synthesize the given compound by a thermal
Concept introduction: A chemical reaction that involves
Answer to Problem 27.40P
The starting materials that are needed to synthesize the given compound by a thermal
Explanation of Solution
The given product is shown below.
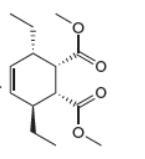
Figure 3
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
The reaction that shows the disconnection approach of the given product is shown below.
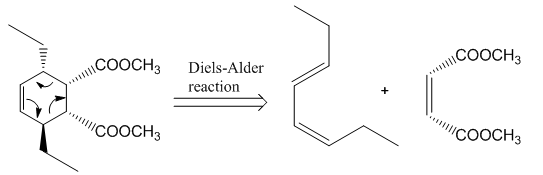
Figure 4
In the given product, the ring is opened due to the rearrangement of
Thus, the starting materials that are needed to synthesize the given compound by a thermal
The starting materials that are needed to synthesize the given compound by a thermal
(c)
Interpretation: The starting materials that are needed to synthesize the given compound by a thermal
Concept introduction: A chemical reaction that involves
Answer to Problem 27.40P
The starting materials that are needed to synthesize the given compound by a thermal
Explanation of Solution
The given product is shown below.
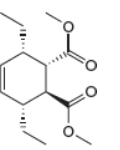
Figure 5
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
The reaction that shows the disconnection approach of the given product is shown below.
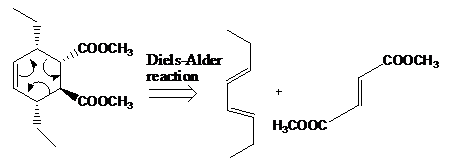
Figure 6
In the given product, the ring is opened due to the rearrangement of
Thus, the starting materials that are needed to synthesize the given compound by a thermal
The starting materials that are needed to synthesize the given compound by a thermal
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry-Package(Custom)
- Draw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forwardProvide the missing information. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
- Provide the missing information. *see imagearrow_forwardI have a bottle of butanal that has been improperly used by lab workers. They allowed a traceamount NaOH (aq) to contaminate the bottle. What is now in my bottle of “butanal? What is the molecular name and functional group name? Draw the structure.arrow_forwardProvide the missing information. *see imagearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

