
(a)
Interpretation:
Chemical structure of the
Concept introduction:
Any polymer can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). Naming of polymer is based on the monomer from which it was derived.
If the monomer contains only one word in the name then no parenthesis is used when naming polymer but if the monomer contains two words means parenthesis is used around the name of monomer.
Monomer with two words=poly(monomer)
Monomer with single word=poly-monomer
(a)
Answer to Problem 22PP
The polymer is polynitroethylene and the structure is
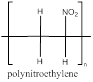
Explanation of Solution
To draw and name the polymer when vinyl acetate undergoes polymerization
Draw the structure of nitroethylene
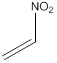
The structure of nitroethylene is drawn following
Polymerization of nitroethylene

The nitroethylene has a double bond which can involve in polymerization with another nitroethylene which propogates to the other molecules to form a chain where the repeating unit is the monomer unit without a double bond as it is involved in polymerization. nitroethylene polymerizes to form polynitroethylene
(b)
Interpretation:
Chemical structure of the polymer should be drawn and named when given monomer undergoes polymerization.
Concept introduction:
Any polymer can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). Naming of polymer is based on the monomer from which it was derived.
If the monomer contains only one word in the name then no parenthesis is used when naming polymer but if the monomer contains two words means parenthesis is used around the name of monomer.
Monomer with two words=poly(monomer)
Monomer with single word=poly-monomer
(b)
Answer to Problem 22PP
The polymer is polyacrylonitrile and the structure is
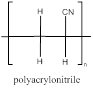
Explanation of Solution
To draw and name the polymer when acrylonitrile undergoes polymerization
Draw the structure of acrylonitrile

The structure of acrylonitrile is drawn following IUPAC nomenclature.
Polymerization of acrylonitrile

The acrylonitrile has a double bond which can involve in polymerization with another acrylonitrile which propogates to the other molecules to form a chain where the repeating unit is the monomer unit without a double bond as it is involved in polymerization. Acrylonitrile polymerizes to form polyacrylonitrile.
(c)
Interpretation:
Chemical structure of the polymer should be drawn and named when given monomer undergoes polymerization.
Concept introduction:
Any polymer can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). Naming of polymer is based on the monomer from which it was derived.
If the monomer contains only one word in the name then no parenthesis is used when naming polymer but if the monomer contains two words means parenthesis is used around the name of monomer.
Monomer with two words=poly(monomer)
Monomer with single word=poly-monomer
(c)
Answer to Problem 22PP
The polymer is poly(vinylidene fluoride) and the structure is
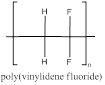
Explanation of Solution
To draw and name the polymer when vinylidene fluoride undergoes polymerization
Draw the structure of vinylidene fluoride
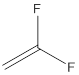
The structure of vinylidene fluoride is drawn following IUPAC nomenclature.
Polymerization of vinylidene fluoride

The vinylidene fluoride has a double bond which can involve in polymerization with another vinylidene fluoride which propogates to the other molecules to form a chain where the repeating unit is the monomer unit without a double bond as it is involved in polymerization. vinylidene fluoride polymerizes to form poly(vinylidene fluoride). As this monomer has two words in its name the polymer is named with paranthesis
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry, Binder Ready Version
- Please predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forward
- If we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forwarddrawing, no aiarrow_forwardIf CH3COCH2CH(OCH3)2 (4,4-dimethoxy-2-butanone) and hydrazine react, two isomeric products are formed. State their structure and which will be the majority.arrow_forward
- + Reset Provide the correct IUPAC name for the compound shown here. 4-methylhept-2-ene (Z)- (E)- 1-6-5-2-3-4- cyclo iso tert- sec- di tri hept hex oct meth eth pent ane yne ene ylarrow_forward+ Provide the correct IUPAC name for the compound shown here. Reset H3C H H C CH3 CH-CH3 1-3-methylpent ene trans- cis- 5-6-3-1-2-4- tert- tri sec- di cyclo iso but pent hex meth prop eth yl ane ene yne ☑arrow_forwarddrawing, no aiarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





