
Concept explainers
(a)
Interpretation:
The E or Z configuration for the following molecule should be determined:
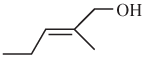
Concept introduction:
(b)
Interpretation:
The E or Z configuration for the following molecule should be determined:
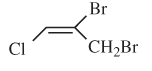
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be named as E and Z-configuration. The E-configuration stands for anti-configuration whereas Z stands for same side configuration. The determination of groups must be done on the basis of their molecular mass. The group or atom with high molecular mass must be numbered as 1 and other with 2. If both 1 numbered group/atom are placed at the same side, they will consider as Z-configuration and in E-configuration these groups will be at anti-position.
(c)
Interpretation:
The E or Z configuration for the following molecule should be determined:
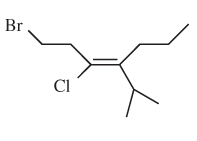
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be named as E and Z-configuration. The E-configuration stands for anti-configuration whereas Z stands for same side configuration. The determination of groups must be done on the basis of their molecular mass. The group or atom with high molecular mass must be numbered as 1 and other with 2. If both 1 numbered group/atom are placed at the same side, they will consider as Z-configuration and in E-configuration these groups will be at anti-position.
(d)
Interpretation:
The E or Z configuration for the following molecule should be determined:
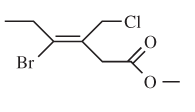
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be named as E and Z-configuration. The E-configuration stands for anti-configuration whereas Z stands for same side configuration. The determination of groups must be done on the basis of their molecular mass. The group or atom with high molecular mass must be numbered as 1 and other with 2. If both 1 numbered group/atom are placed at the same side, they will consider as Z-configuration and in E-configuration these groups will be at anti-position.
(e)
Interpretation:
The E or Z configuration for the following molecule should be determined:
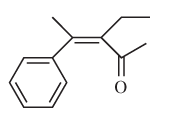
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be named as E and Z-configuration. The E-configuration stands for anti-configuration whereas Z stands for same side configuration. The determination of groups must be done on the basis of their molecular mass. The group or atom with high molecular mass must be numbered as 1 and other with 2. If both 1 numbered group/atom are placed at the same side, they will consider as Z-configuration and in E-configuration these groups will be at anti-position.
Trending nowThis is a popular solution!

Chapter 26 Solutions
GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
- Consider the molecule 1-bromo-2-methylbutane. C3 and C4 should be drawn as Et as in theexample. This group is called an ethyl group and can be considered a sphere about twice the sizeof a methyl group. Draw the following Newman projections sighting down the C1C2 bond... a. The lowest potential energy conformation. b. The highest potential energy staggered conformation.arrow_forwarddraw and name two structures that match the description of a trans-dihalocyclopentanearrow_forwardI don't understand how to tell which one goes on which side. When I looked at the answer key to check my answer, I had H/Br and H/NH2 on the wrong sidesarrow_forward
- ball & stick v + labels Which of the Newman structures below represents this conformation of 3-chloro-2-methylpentane, as viewed along the C2-C3 bond? ***** CH3 Et H Et Et. .CI H3C. CH3 Et. H3C. H3C H. TCI H3C CH3 H. ČH3 H ČH3 a b d (Enter the letter(s) of the correct structure(s), in alphabetical order and without punctuation; more than one answer may be correct. Et = an ethyl group)arrow_forwardIt is easy to imagine a cyclohexane as a flat hexagon and a lot of the time we draw it that way. Looking at 1,3,5-triethylcyclohexane we cannot tell the stability of the molecule from looking at the flat 2D drawing. Explain why we need to look at the 3D configuration and what conformation (axial,equatorial) would each of the three ethyl groups be in for the most stable configuration.arrow_forwardWhich of the following represents the LUMO for cyclopentadiene?arrow_forward
- A Newman projection of methylcyclohexane is shown below. List the types of strain present in this conformation. H. H. H. H2 CH3 C H2 H.arrow_forwardSome of the following examples can show geometric isomerism, and some cannot. Forthe ones that can, draw all the geometric isomers, and assign complete names using theE-Z system. cyclohexene cyclodecenearrow_forwardBenzene is especially stable due to... O the electrons of the double bonds are delocalized O its planar shape the total number of carbors in the molecule each carbon has four bonds « Previousarrow_forward
- Write a structure ( Chair conformation )arrow_forwardFor which isomer would you expect a greater equilibrium percentage of molecules with the alkyl group in the axial position, explain.arrow_forwardWhich of the following molecules has the lowest strain energy? Cyclobutane Cyclopentane Cyclopropane Cyclohexanearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
